Building bridges in education: Exploring how engineering and MBA students can work together to help the environment using biodiesel
The growing demand for alternative fuels has propelled biodiesel into the spotlight as a viable solution. This case study at Manhattan College exemplifies interdisciplinary collaboration between chemical engineering and business students. It focuses on transforming waste vegetable oil into biodiesel, offering an eco-friendly fuel source and glycerin for soap and candle production. MBA students conducted a feasibility analysis for these products. Promising applications emerged: Biodiesel for generators, glycerin-based soap, and candles. This experience equips students with practical skills and emphasizes the value of engineering-business partnerships in education, encouraging cross-disciplinary teamwork and sustainable innovation.
1 Introduction
As the world grapples with the mounting economic and environmental costs associated with traditional fossil fuels and climate change caused by fossil fuel combustion, the urgency to explore alternative energy sources becomes ever more apparent (Maleki et al., 2023). Among these alternatives, biodiesel emerges as a promising contender, offering a sustainable substitute for petroleum-based fuels. Derived from natural feedstocks like vegetable oil, biodiesel presents a versatile solution to the energy crisis. It’s not only capable of operating independently from petroleum but can also be blended with it, thereby mitigating the challenges posed by temperature-induced viscosity variations. This versatility is demonstrated through various blends, such as B2 (two percent biodiesel/ninety-eight percent petroleum), B5 (five percent biodiesel/ninety-five percent petroleum), B20 (twenty percent biodiesel/eighty percent petroleum), and the pure B100. Among these, B20 stands out as the most popular, as it ensures optimal engine power retention (Jamashaid et. al., 2022).
Biodiesel exhibits impressive energy output and shines as a cleaner-burning fuel, leading to substantially reduced carbon emissions compared to conventional petroleum. For instance, the deployment of a B20 blend can slash carbon emissions by a notable fifteen percent, highlighting its potential in combating environmental degradation (nrel.gov).
The cornerstone of biodiesel production is the transesterification reaction (see Figure 1), a transformative process that converts waste vegetable oil into biodiesel and glycerin. This reaction involves the introduction of liquid alcohol, typically methanol or ethanol, alongside a base like sodium hydroxide or potassium hydroxide, to the vegetable oil. A valuable byproduct of this reaction is glycerin, which opens doors to diverse applications, including the production of soaps, candles, and various other products.
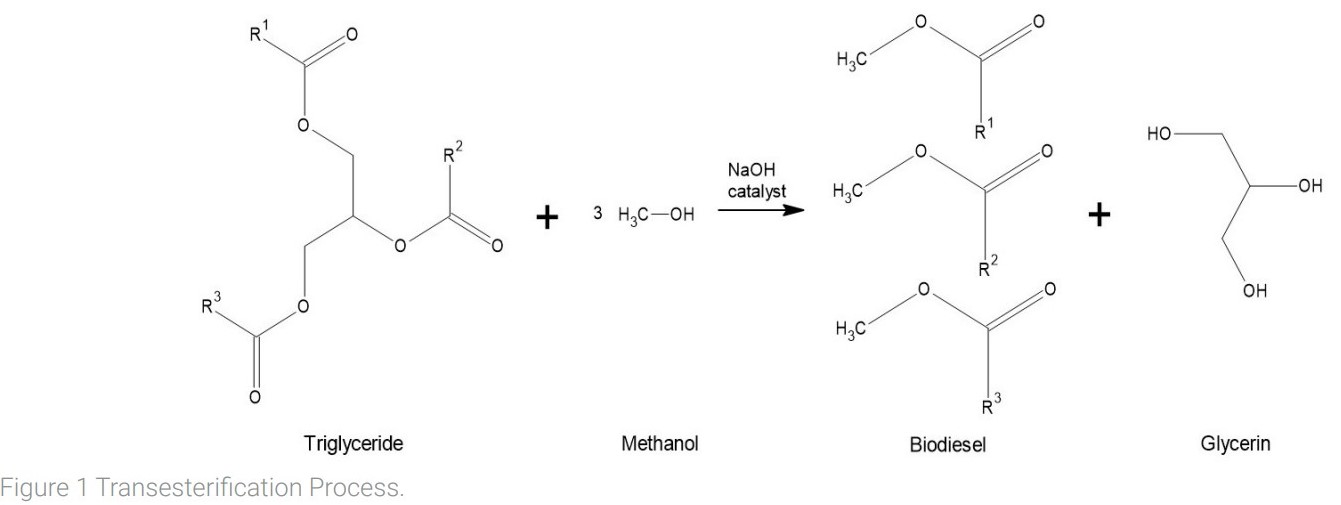
Once the transesterification reaction is concluded, the extracted biodiesel requires refinement to achieve optimal purity. One purification technique involves using water, capitalizing on the non-polar nature of biodiesel molecules. Water’s polarity attracts and entraps impurities, effectively separating them from the biodiesel as the two liquids separate. This washwater, laden with contaminants, is subsequently separated through phase separation. Alternatively, the application of Magnesol D-Sol, a synthetic magnesium silicate powder, offers another impurity removal avenue. This innovative method involves suspending the filtering powder within the biodiesel, where it actively binds to impurities. However, the powder itself remains suspended and necessitates an additional filtration step for its complete elimination (Berrios and Skelton, 2008).
The feedstock for biodiesel production predominantly comprises vegetable cooking oil, although other sources can also be employed. Examples encompass plant-based oils such as peanut oil, animal oils derived from animal fats, and a wide array of cooking oils (Singh et al., 2014). These sources share the presence of long fatty acid chains and esters, which can be skillfully converted into biodiesel. While certain alternative oils may possess a higher concentration of these molecules than vegetable oil, the latter remains the preferred choice due to its abundance and cost-effectiveness.
Biodiesel transcends its role as a mere alternative to petroleum, representing a dynamic solution poised to revolutionize the energy landscape. Its versatility, efficiency, and environmentally conscious nature position it as a key player in the pursuit of sustainable energy sources. To get new engineers to think about their role in the environment and sustainability, colleges can help. Colleges serve as fertile grounds for exploring the intricate dynamics of product manufacturing and assessing their economic viability due to their conducive environment for interdisciplinary collaboration, robust research infrastructure, and the inherent pursuit of knowledge-driven innovation. Engaging students in real-world projects involving the conceptualization, development, and production of various products not only facilitates hands-on learning but also encourages critical thinking and problem-solving skills. The convergence of diverse academic disciplines, ranging from engineering and business to environmental studies and chemistry, provides a rich tapestry of perspectives that enriches the process of product ideation and evaluation. This multidimensional approach equips students with a holistic understanding of the complex interplay between technical feasibility, market demand, resource availability, and cost considerations.
In the context of biodiesel production, for instance, colleges can harness their academic prowess to examine every facet of the process, from sourcing feedstock to refining end products. Through interdisciplinary collaborations, engineering students can leverage their technical expertise to optimize the production process, while business students can apply economic models to assess the financial feasibility of the venture. Moreover, the vast array of research facilities, laboratories, and faculty expertise available within academic institutions enables comprehensive analyses of product attributes, quality, scalability, and environmental impact. The academic philosophy of inquisitiveness and empiricism propels students to not only develop innovative solutions but also rigorously evaluate their potential success in real-world contexts. By immersing students in such experiential learning endeavors, colleges can impart valuable skills that empower future professionals to navigate the intricate nexus of product creation and economic viability, while fostering a culture of sustainable innovation within academia and beyond.
2 Prior ventures
Embedded within the broader sustainability movement sweeping the nation, biodiesel production has emerged as a pivotal element of environmental responsibility. This movement has found resonance within numerous colleges and universities, fostering the adoption of green methodologies to reduce ecological footprints. Emblematic of this trend is Manhattan College, a proactive participant in this wave of change. The college has undertaken research into biodiesel production and its pragmatic integration of both biodiesel and glycerin derivatives within its campus ecosystem. Echoing this commitment, other distinguished institutions like Tufts University, the University of Missouri, and Pennsylvania State University are making strides in biodiesel research.
2.1 Tufts University’s Sustainability Program:
At Tufts University, a comprehensive sustainability program is at the forefront of their agenda. Biodiesel holds a prominent position within their efforts, not just within the campus borders, but with aspirations to extend its impact beyond. The university’s investigation into biodiesel encompasses its application in university vehicles, thereby aligning with their sustainability objectives (sustainability.tufts.edu). Furthermore, Tufts has since ventured into the international realm, proposing a biodiesel production scheme in Turkey aimed at mitigating global warming (gordon.tufts.edu). This initial proposal evolved into an ambitious business plan, meticulously scrutinized for its marketing, operational, and financial viability. While substantial strides have been taken, a comprehensive examination of biodiesel’s efficiency and cost-effectiveness as an alternative fuel, and the potential to integrate its benefits across campus activities, remains to be explored.
2.2 University of Missouri’s Focus on Biodiesel Innovation:
Dr. Schumacher’s research endeavors at the University of Missouri have placed biodiesel at the forefront of innovation. His emphasis on soybean oil as a feedstock for biodiesel production has led to insightful revelations (Schumacher et al., 1993). Through his research, he gauged the quality differences between various biodiesel blends and explored their potential in powering heavy-duty diesel engines (Schumacher et al., 1994). Notably, Schumacher delved into the realm of public transportation, with a particular emphasis on buses (Schumacher and Weber, 1994; Chandler et al., 1996). Emissions reductions from diverse engines, such as the Series 60 DDC engine (Schumacher, 1995) and the Cummins L10E engine (Marshall et al., 1995), were scrutinized, underlining biodiesel’s potential in curbing pollution. However, while the research has shed light on the biodiesel-bus nexus (Schumacher and Gerpen, 2000), its scope has yet to encompass the broader implications of biodiesel across the entirety of the university’s operations.
2.3 Pennsylvania State University’s Pilot Plant Innovation:
The Pennsylvania State University’s commitment to biodiesel innovation shines through its dedicated biodiesel pilot plant, tailored for batches of 25 gallons. This facility serves as a hub for multifaceted research, exploring areas such as biodiesel reactions at room temperature, methanol recovery, and the application of novel technologies like thermoelectric condensers and small-scale reactors. Their explorations have even encompassed the intricacies of biodiesel quality, pour point dynamics, and flash point analyses. Notably, the university’s foray into alternative feedstocks, such as camilina oil, displays a quest for innovation. Although the university’s intention to leverage waste cooking oil from its cafeterias for biodiesel production is evident, a comprehensive blueprint for implementation remains a work in progress (che.psu.edu).
In sum, these academic institutions exemplify the synergy between education, research, and sustainability in the realm of biodiesel production. Their endeavors underscore the transformative potential of biodiesel as a key catalyst in advancing environmental consciousness within higher education institutions. While strides have been made, there lies untapped potential in comprehensively integrating biodiesel’s benefits across campus-wide operations and exploring its feasibility beyond specialized applications.
3 Execution
With a vision to empower communities, two chemical engineering students embarked on a journey to create a viable blueprint capable of yielding abundant biodiesel energy for diverse applications. Drawing upon both virgin vegetable oil and discarded culinary oils from the college’s cafeterias, the students engaged in a partnership with the Mechanical Engineering department. Collaboratively, they harnessed the power of a Lister engine to assess the efficiency of both virgin and waste-based biodiesel, measuring their prowess against conventional petroleum fuel. Their studies ventured into the realm of biodiesel purity and fuel efficiency, navigating the complex interplay of varying alcohols and bases in the biodiesel synthesis process.
Building on this, another project, undertaken by four chemical engineering students focused on the transformation of glycerin, a byproduct of the biodiesel production process, into an array of utilitarian commodities. They envisioned the creation of bar soaps, liquid soaps, and candles. As the project’s trajectory evolved, it naturally dovetailed into a harmonious collaboration between the chemical engineering undergraduates and their counterparts from the business school. At a college/university that houses business and engineering schools, faculty members across the two were able to introduce students in their respective classes to this project with relative ease. Specifically, two faculty members (one from chemical engineering and one from the MBA program) provided support for students by allowing them time to meet to work with their counterparts. Together, this interdisciplinary brigade of students undertook the task of crafting an intricate cost and feasibility analysis for the array of products stemming from the biodiesel reaction. These products, poised on the cusp of realization, carried the potential to resonate with a spectrum of stakeholders within the college’s vibrant ecosystem.
The significance of Manhattan College’s biodiesel initiative became ever more pronounced in the aftermath of the catastrophic Hurricane Sandy’s assault on New York City. Amid the storm’s fury, the rending of a power line rendered two campus buildings powerless, one of them a dormitory. Cast into darkness, the dormitory’s lifeline rested upon backup generators. However, the generator’s roar came with a toll, both logistical and financial. Resource scarcity in the hurricane’s wake, coupled with the hefty operational costs of a generator at full throttle, cast a shadow over the students’ experience, depriving them of in-room power for weeks. It was in this pivotal moment that the potential of the biodiesel initiative truly came to the forefront. The conceptualized plan to harness biodiesel for the generators, forged with foresight, could have been a transformative force during the storm’s aftermath, fostering uninterrupted energy supply as the campus rebuilt. Instead, the college found itself compelled to procure conventional fuel, relinquishing the opportunity to leverage its culinary discards for a sustainable solution.
4 Findings
The initial inquiry into the potential of biodiesel production was conducted to ascertain its applicability within the institutional context of the college. A significant benefit materialized in the form of addressing waste vegetable oil disposal from the college cafeterias. This investigation illustrated that the current practice of engaging external entities for oil disposal could be reduced, if not eliminated, by utilizing the cafeterias’ used cooking oil. Consequently, an immediate and tangible financial gain is envisaged through the abrogation of disposal costs. Moreover, the adoption of biodiesel production from spent cooking oil holds the promise of curbing oil accumulation in landfills, thereby fostering a waste-reduction framework.
A feedstock analysis was next performed to forecast the expected outputs from the biodiesel process. This analysis revealed that the quartet of cafeterias at Manhattan College collectively yields 220 gallons of waste vegetable oil per month. Assuming a conversion efficiency of approximately 70%, this translates to the monthly production of 150 gallons of biodiesel. Over the course of seven operational months, this aggregated to 1050 gallons of biodiesel, destined for application in campus vehicles or as heating oil. The residual 70 gallons are allocated for conversion into glycerin, which holds potential for the creation of diverse commodities such as bar soap, liquid soap, and candles.
The initial phase of application assessment concentrated on the feasibility of employing biodiesel for campus vehicles. Despite its merits, this avenue was constrained by the recent acquisition of gasoline-fueled vehicles by the security department, rendering plans for an immediate switch to diesel-fueled vehicles unviable.
The next application was the use of heating oil for the backup generators. With 1050 gallons of biodiesel available, it was determined that the generators at all four of the dormitories would be able to run at the same time at full capacity for at least two days, continuously. During unfortunate and unforeseeable events such as Hurricane Sandy, this prospect holds the potential to yield substantial cost savings. By blending biodiesel with conventional petroleum fuel, an extended operational period for the generators can be realized, resulting in concurrent reduction of operational expenses. The favorable economics of producing biodiesel from cooking oil, when contrasted with procuring petroleum diesel, substantiates the feasibility of this application.
Bar soap was another application of the biodiesel process that was analyzed. Bar soap is a product of a saponification reaction where glycerin is reacted with sodium hydroxide to make soap. In order to make bar soap from the glycerin, the glycerin needs to be purified from any excess methanol before it can be molded. Because methanol has a low boiling point of 65°C (or 149°F), heating up the glycerin beyond this temperature will effectively remove the methanol. The issue lies in the use of sodium hydroxide. Even though sodium hydroxide is the necessary reactant, it does not react as well with glycerin as it would with vegetable oil. The resources required in both time and materials to produce an acceptable quality solid bar of soap indicated that the break-even point was beyond the capacity of the school (i.e. the cafeterias would not be able to provide enough used vegetable oil to make this product profitable).
After analyzing bar soap, liquid soap was analyzed for feasibility. Liquid soap also goes through a saponification reaction as did the bar soap once the methanol is removed. For liquid soap, however, potassium hydroxide is used in the reaction as opposed to the sodium hydroxide used for bar soap. When the saponification reaction is completed, the products are further cooked to maintain the soap in liquid state. In the feasibility analysis, the liquid soap would be used to stock the soap dispensers throughout the campus buildings. There are a total of 75 soap dispensers on campus which require 172 cases of soap containing six liters of soap each per year. Each case costs the school $42.50 which makes the total cost of soap $7,310 per year for the campus. The ratio of reactants to product made was scaled up to account for the total amount of soap needed to provide the college with soap for a year. The relevant data for the cost analysis is shown in Table 1.
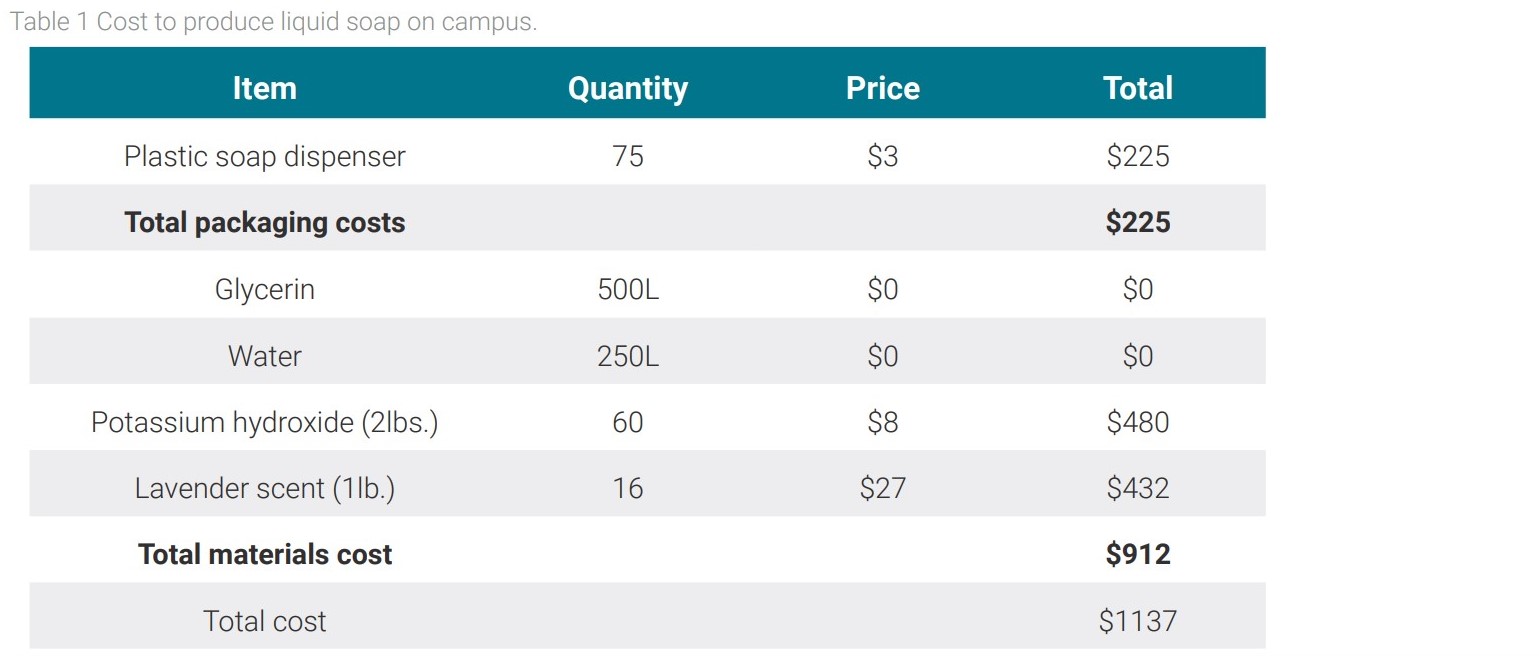
Based on this table, the total cost of manufacturing liquid soap from glycerin comes out to be $1,137, approximately one seventh of the cost of buying liquid soap each year. This option saves the college $6,173 per year. After five years the college will end up saving over $30,000. One issue arises from the fact that the current method for making the soap takes 45 minutes from start to finish and produces only three liters of soap. One proposed solution is for the college to hire its students under work-study to produce the soap. The cost of hiring students to make soap is shown in Table 2.
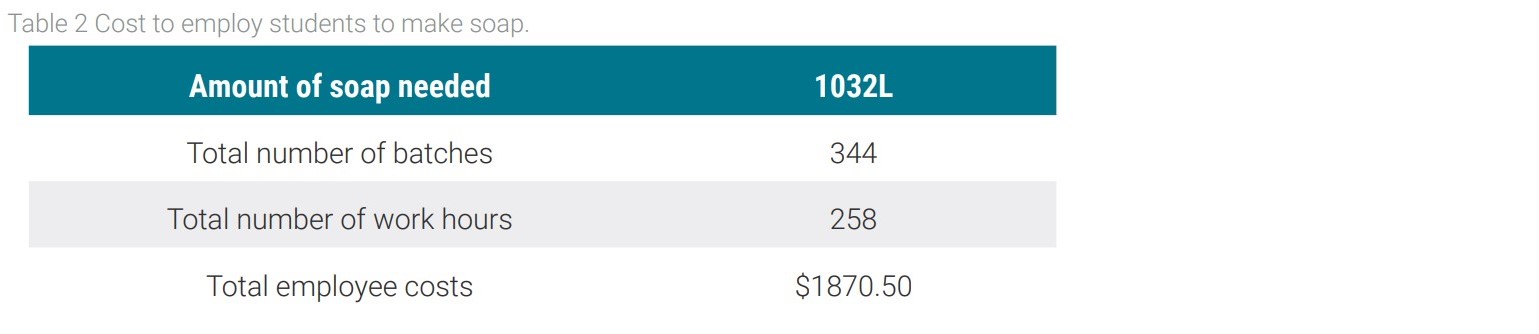
From the table above, the total cost of hiring students to produce the needed soap from the school comes out to $1870.50. Even with this additional cost, the final cost to produce soap for the whole campus is $3,007.50. With this option the college still saves $4,302.50 per year, making it an extremely feasible option.
The last option in the feasibility study was the manufacturing of candles. The components of a candle include the wax, wick, color, fragrance, and jar, which is more material than what goes into making any of the other biodiesel derivatives. To make the wax, the glycerin has to be melted down to a liquid. Color in the form of either a pigment or a dye along with the fragrance is added to the melted glycerin. The wick is then placed into the jar and the wax is poured in and set aside to cool. A major obstacle in the candle venture is that they are not allowed in any of the dormitories at this school as they are deemed a fire hazard. As a result, the manufactured candles would only be sold on holidays or before breaks. Based on the typical costs of materials for making candles, a cost analysis is shown in Table 3.
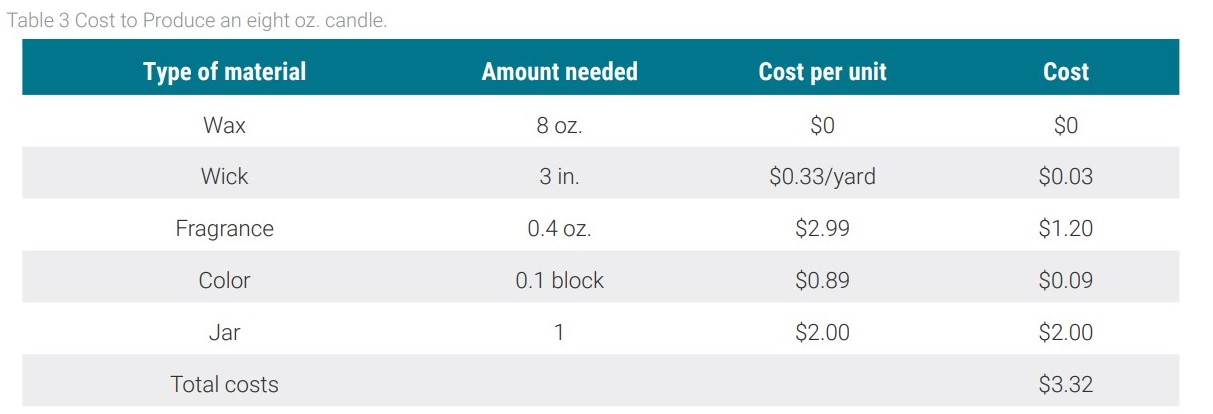
Assuming that the candles being manufactured are the smallest typical size, eight oz., the total cost to manufacture candles would be $3.32 per candle. If the candles were sold at $5.00 per candle there would be a profit of $1.68 per candle. It was determined that a total of 2586 candles would be needed every year but with supply of glycerin from the biodiesel process, only 1584 candles can be made every year. Assuming that the candles would be sold at $5 a candle the total revenue from this option is $2661.12. This option turns out to be a feasible option that can provide the school with a profit of $2661.12.
5 Conclusion
The biodiesel production endeavor at Manhattan College served as a multifaceted platform, yielding not only biodiesel but also an array of valuable commodities extracted from the glycerin byproduct. In light of this, an exhaustive feasibility analysis was meticulously undertaken to ascertain the optimal utility of both biodiesel and glycerin. The scope of potential applications encompassed utilizing biodiesel for campus vehicles, employing it as heating oil for backup generators, and harnessing the glycerin byproduct to create bar soap, liquid soap, and candles. The ensuing feasibility assessment was not confined solely to demand dynamics, but also intricately accounted for the raw material supply, manufacturing expenses, and underlying production processes inherent to each application.
Investigation into the viability of biodiesel for campus vehicles revealed an untenable proposition due to the recent replacement of the college’s vehicle fleet with gasoline-powered models. In contrast, the notion of utilizing biodiesel as heating oil for generators emerged as a substantiated and practicable endeavor. The extant reservoir of vegetable oil derived from cafeterias gives ample potential to generate sufficient biodiesel capable of powering all on-campus generators uninterruptedly for a duration of two days. This proactive approach to energy provisioning is poised to set up substantial fuel cost savings, particularly during exigencies such as the Hurricane Sandy scenario, characterized by escalated fuel costs and limited supply.
Regarding the biodiesel byproducts stemming from glycerin, the feasibility analysis showed the nonviability of bar soap due to protracted processing times and inconsistent product quality. In contrast, the production of liquid soap emerged as a promising avenue. The liquid soap manufactured from the supply of glycerin would be enough to supply all the soap dispensers at Manhattan College each year. The annual yield of liquid soap, derived from the available glycerin supply, could effectively replenish all soap dispensers across Manhattan College. The temporal constraints inherent to soap manufacturing could be assuaged by engaging students under a work-study model, a stratagem that, despite incurring labor costs, ultimately realizes significant annual savings exceeding $4,000.
Conversely, the feasibility of candle manufacturing revealed incongruities in demand and supply dynamics, rendering the enterprise unfeasible. While the production cost per candle stood at $3.32, indicating potential profitability, the incongruity between the estimated supply of 1584 candles and the projected demand of 2586 candles underscored a notable demand deficit. Considering this demand-supply disparity, revenue projections for candles remained less promising.
A crucial facet of the biodiesel production project lay in the synergistic collaboration between the business school and the chemical engineering department. This interdisciplinary engagement facilitated a holistic real-world analysis. The assessment of biodiesel production extended beyond mere yield considerations, encompassing the practicality of each derivative. This symbiotic relationship gleaned mutual benefits, empowering engineers with practical business insights and endowing business students with technical acumen. Such partnerships can be effectively replicated within other institutions, wherein chemical engineering faculty can align with their business counterparts. Such collaborative initiatives could manifest as independent research endeavors akin to those delineated in this study or be seamlessly integrated into curricula, particularly within courses such as plant design enriched with an economic dimension. The cultivation of interdisciplinary interactions holds potential not only to enrich institutional prospects but also to augment the skill set of participating students.
6 Future work
In light of the delineation of the most viable biodiesel products, the imperative to formulate an actionable plan becomes manifest. Pertaining to the projected yield of 1050 gallons of biodiesel earmarked for serving as heating oil for the generators, a series of strategic determinations come to the fore. Foremost among these determinations is the selection of an appropriate storage facility for the biodiesel during periods of dormancy. With the assumption that power disruptions will be infrequent, the entirety of the 1050-gallon yield necessitates simultaneous storage provisioning. Moreover, the attendant cost of establishing and maintaining such storage infrastructure mandates meticulous evaluation.
Along with this, the establishment of a cogent production and delivery schedule emerges as a paramount requirement. This schedule should be intricately synchronized with the anticipated removal of used cooking oil from the campus cafeterias. Furthermore, to ensure a steady and sufficient supply of liquid soap at times of demand, the formulation of a comprehensive production schedule for liquid soap attains significance. Similarly, the production of candles warrants a calendrical outline that accommodates the provisioning of ample candles for students’ acquisition ahead of the impending holiday season and the culmination of academic sessions.
In a prospective trajectory, the reevaluation of the feasibility of integrating diesel-powered campus vehicles within the institution’s transportation ecosystem remains an intriguing proposition. This future course of action may entail a comprehensive reassessment of the prevailing campus vehicle fleet, taking into account evolving technological advancements and potential shifts in logistical paradigms. An alternative to implementing biodiesel in security vehicles is to use the fuel in less urgent vehicles such as with physical plant services.
In essence, the delineated biodiesel initiatives not only hinge on the identification of feasible products but also necessitate the formulation of meticulously orchestrated operational strategies that span across various temporal horizons. Such strategic orchestration is intrinsic to ensuring optimal utilization of resources, maximizing the realization of projected benefits, and fostering a sustainable integration of biodiesel-based applications within the campus environment.
References
Berrios, M. & Skelton, R.L. (2008): Comparison of purification methods for biodiesel. Chemical Engineering Journal, 144 (3), pp. 459-465.
Chandler, K., Malcosky, N., Motta, R., Norton, P., Kelly, K., Schumacher, L., & Lyons, D. (1996): Alternative fuel transit bus evaluation program results. SAE transactions, pp. 609-633.
Jamshaid, M., Masjuki, H.H., Kalam, M.A., Zulkifli, N.W.M., Arslan, A., & Qureshi, A.A. (2022): Experimental investigation of performance, emissions and tribological characteristics of B20 blend from cottonseed and palm oil biodiesels. Energy, 239, pp. 1-15.
Maleki, B., Singh, B., Eamaeili, H., Venkatesh, Y.K., Talesh, S.S.A., & Seetharaman, S. (2023): Transesterification of waste cooking oil to biodiesel by walnut shell/ sawdust as a novel, low-cost and green heterogeneous catalyst: Optimization via RSM and ANN. Industrial Crops & Products, 193, pp. 1-18.
Marshall, W., Schumacher, L.G., & Howell, S. (1995): Engine exhaust emissions evaluation of a Cummins L10E when fueled with a biodiesel blend. SAE Technical Paper.
Schumacher, L.G., Borgelt, S.C., & Hires, W.G. (1993): Soydiesel/petroleum blend research. American Society of Agricultural Engineers. Chicago, IL.
Schumacher, L.G., Borgelt, S.C., Hires, W.G., Fosseen, D., & Goetz, W. (1994): Fueling diesel engines with blends of methyl ester soybean oil and diesel fuel.
Schumacher, L.G. & Gerpen, J.V. (2000): Engine oil analysis of diesel engines fueled with 0, 1, 2, and 100 percent biodiesel. American Society of Agricultural Engineers. Milwaukee, WI.
Schumacher, L.G., Marshall, W., Krahl, J., Wetherell, W.B., and Grabowski, M.S. (2001): Biodiesel emissions data from series 60 DDC engines. Transactions of the ASAE, 44 (6), pp. 1465-1468.
Schumacher, L. G. & Weber, J.A. (1994): Collection and collation of performance data from urban mass transit biodiesel demonstrations. American Society of Agricultural Engineers. Kansas City, MO.
Singh, B., Guldhe, A., Rawat, I., & Bux, F. (2014): Towards a sustainable approach for development of biodiesel from plant and microalgae. Renewable and Sustainable Energy Reviews, 29, pp. 216-245.