Carbon Capture and Utilization – A new building block for Circular Economy?
Abstract
Reducing the emissions of greenhouse gases is a great challenge for today’s industry. Besides using efficiency improvements and CO2 free processes, the utilization of CO2 as a raw material could be an important technology on the way to a greenhouse gas neutral world. Especially in the chemical industry these processes could help to implement a fully circular economy. For now, Carbon Capture and Utilization (CCU) is in its infancy and just a few demonstration plants are already running. After introducing some CCU processes, this article shows the hurdles and potentials of CCU in general. Knowing the hurdles of this new technology is important to accelerate the implementation in an efficient way. The potentials demonstrate how influential CCU could be towards a circular economy for the chemical industry. The basis for this work is the content analysis of expert interviews. The interviews were held with professionals from the chemical industry.
Introduction
Climate change is one of the greatest threats to humanity. To avert this crisis, the Intergovernmental Panel on Climate Change (IPCC) has set the target to stay below a 2 °C increase of the global temperature (United Nations, 2015). Following the recommendation of the IPCC, governments and companies worldwide have set their own CO2 reduction targets. The European Union (EU) wants to be climate neutral until 2050. An important milestone is the year 2030, when the EU aims to have lowered the output of CO2 by 55 % compared to 1990 (European Commission, 2020b).
Today companies are under societal and political pressure to reduce their CO2 output. By introducing the Emission Trading System (ETS) the EU has set up a monetary incentive for companies to reduce their CO2 output (Directive 2003/87/EC, 2003). Since modern industries rely on fossil fuels that inevitably produce CO2 , energy intensive companies need innovative solutions to drastically reduce their CO2 output.
Carbon Capture and Sequestration (CCS) may be a solution, since large amounts of CO2 could be stored in geological sites (Gibbins & Chalmers, 2008). But stored CO2 could lead to diffuse leaks, that are hard to handle. Furthermore, the public perception of CCS is negative. Therefore, CCS on an industrial scale is forbidden in the EU (Directive 2009/31/EG, 2009).
A similar technology is Carbon Capture and Utilization (CCU). By using captured CO2 to manufacture goods it can substitute fossil resources and bind CO2 in products for a certain duration. Consequently, the output of CO2 into the atmosphere is lowered (Assen et al., 2013). In contrast to CCS, CCU can produce added value for companies, making it a better alternative economically. Due to the manifold applications of CCU, it is an interesting option for the chemical industry (Baena-Moreno et al., 2019). Substituting fossil resources with CO2 could decouple the industry from oil and gas imports and therefore pave the way towards circular economy (Kätelhön et al., 2019). As the societal pressure on companies rises and fees for CO2 outputs increase, many companies develop systems to reuse CO2 to generate a higher added value. Currently, using CO2 to produce chemical goods is more complex than the conventional alternative. Higher costs and rejection by industry managers is the consequence. Furthermore, there are regulatory barriers. For example, there is no exception for CCU in the EU ETS (Implementing Regulation (EU) 2018/2066, 2018). So, CO2 that is bound in products counts as emitted and companies need to buy certificates accordingly.
To efficiently implement CCU as technology that promotes circular economy in the chemical industry it is important to identify and understand its potentials concerning economic and technologic issues and aspects of climate protection. On the other side, its hurdles need to be recognized to enable companies to manage them. Both scopes of CCU are significant, as an ideal circular economy has to be sustainable from an economic and a societal point of view (Kirchherr et al., 2017).
In this paper application potentials of CCU will be examined. Therefore, a range of CCU technologies will be discussed. A special focus will be put on applications that have a high impact for circular economy. Relevant hurdles for CCU that prevent an implementation on industrial scales will be reviewed in economic, technologic and regulatory dimensions. To generate a holistic picture of CCU, an analysis of the scientific literature and the knowledge of industry experts is combined.
2 Theoretical Background
2.1 Chemical Industry
The chemical industry is one of the most energy-intensive process industries. In Germany, the sector accounted for 28,6 % of total industrial energy consumption in 2018. However, it is important to note, that more than one third of energy carriers in the chemical industry are used as raw materials for chemical syntheses (Statistisches Bundesamt, 2021). This is a peculiarity of the chemical industry and leads to two problems concerning its CO2 emissions.
The chemical industry has an intrinsic demand for carbon, as numerous chemical products are based upon the carbon atom. Hence, the chemical industry is forced to use carbonaceous resources. Utilization of fossil resources is established as the major source of the chemical industry, while biomass is only used as a minor resource (European Chemical Industry Council, 2019). Synthesized chemical products based on fossil resources decompose at the end of their product life cycle and release CO2 into the atmosphere. Therefore, they contribute to climate change. Products that are based on biomass would also release CO2, but the emissions are balanced by CO2 sinks.
The chemical industry has a potential to reduce its CO2 emissions that is special to this industry. Many products can be built out of CO2 and other renewable resources like H2. So, CO2 can be used as a resource for the chemical industry. Production processes can be transformed from today’s fossil dependency to a more sustainable alternative. Using CO2 as major carbonaceous resource can reduce the carbon footprint of chemical products significantly, depending on the CO2 source (Assen & Bardow, 2014). By using CCU the demand for carbon of the chemical industry could turn into a carbon cycle.
The second problem of the chemical industry is that chemical processes consume a lot of energy. As renewable energy sources do not account for a significant share of used energy carriers in the industry, the high energy consumption leads to high CO2 emissions. Despite efficiency gains, total CO2 emissions did not drop in the last years because of rising production volumes (European Chemical Industry Council, 2019). Innovations that reduce the CO2 intensity of chemical production processes often come with a rising energy intensity. This means that the transformation of the chemical industry towards a lower CO2 intensity in the production process comes with an even higher energy intensity (Geres et al., 2019).
2.2 Emissions Trading
Following the ambitious goals for climate protection in the EU, there are several mechanisms to reduce CO2 emissions. The most important instrument is the EU emissions trading system (Directive 2003/87/EC, 2003). It is a “cap and trade” mechanism. Emissions of all plants that are included in the ETS are capped by the EU. To emit a ton of CO2 a plant has to return certificates. As the certificates are capped, they receive a real exchange value. Certificates can be traded by companies and member states of the EU. But every company and state has to buy enough certificates for their emissions. The total amount of certificates has been reduced by 1,74 % per year until 2020 (Directive 2009/29/EG, 2009). Since 2021 the reduction rate rises to 2,2 % per year to motivate companies to install sustainable processes even faster (Directive (EU) 2018/410, 2018).
The EU ETS is the world’s biggest market for greenhouse gas certificates. It accounts for approximately 45 % of greenhouse gas emissions in the EU. Since 2013 many plants of the chemical industry have been taken into account in the EU ETS. In recent years certificates have been traded for prices between 20 € and 50 € per ton of CO2 (Ember, 2021). This leads to high costs for the chemical industry as it is dependent on fossil fuels. The emerging economic problem will enlarge in the future given that certificate prices are going to further rise in the future.
To dampen the costs for the European industry, some certificates are allocated to energy intensive plants for free. To be eligible to get free certificates companies have to produce with a low CO2 intensity. Productions are measured by a benchmark. Top 10 % of the most efficient plants compose the benchmark for their industry sector. Using these benchmark-plants the number of free certificates is calculated. If plants meet the requirements of the benchmark, they get free certificates. But the total number of free certificates decreases over time to promote less CO2 intense processes in all industry sectors. In 2020 only 30 % of all certificates have been allocated for free. Furthermore, the carbon leakage mechanism has been implemented to prevent the relocation of companies into foreign countries without an ETS. Plants that are entitled to the carbon leakage mechanism still get 100 % of their certificates for free (Delegated Regulation (EU) 2019/331, 2018).
2.3 Circular Economy
If the global population continues to grow to more than 9 billion people by 2050 and the economy still produces with today’s process routines, we would need resources equal to three planets to continue our lifestyle (United Nations, 2020). The consumption of basic resources like biomass, fossil fuels, metals and minerals could double in the next 40 years (OECD, 2019). Using the production routines of a circular economy could help to reduce the consumption of resources.
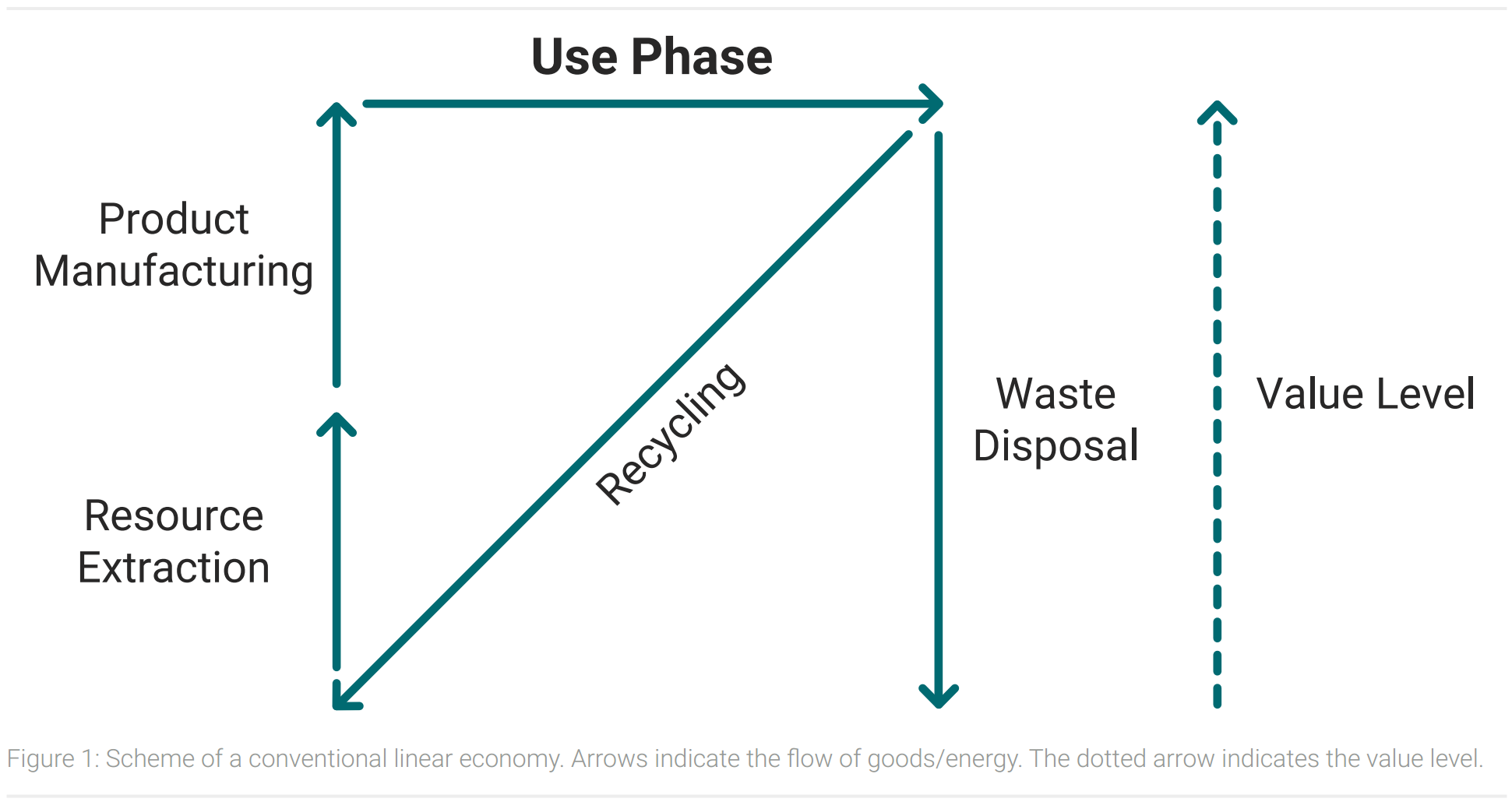
There is a big difference between an ideal circular economy and today’s economy. The conventional economy roughly consists of four processes. Resources are extracted, processed to products, used and disposed as waste after the product lifecycle. The amount of waste this system produces will increase in the next years and could rise up by 70 % by 2050 (Kaza et al.). Figure 1 shows a schematic illustration of today’s linear economy combined with the concept of a value level.
Final products have a higher value level than their raw materials, as resources and energy have been used for the production. In developed countries a significant share of waste is recycled, but products of recycling processes are often raw materials. Their value level is equally low to raw materials because resources and energy have to be put in to produce final products. So recycling reduces the consumption of primary raw materials, but other resources and energy are still used to produce final goods for consumers (Korhonen et al., 2018).
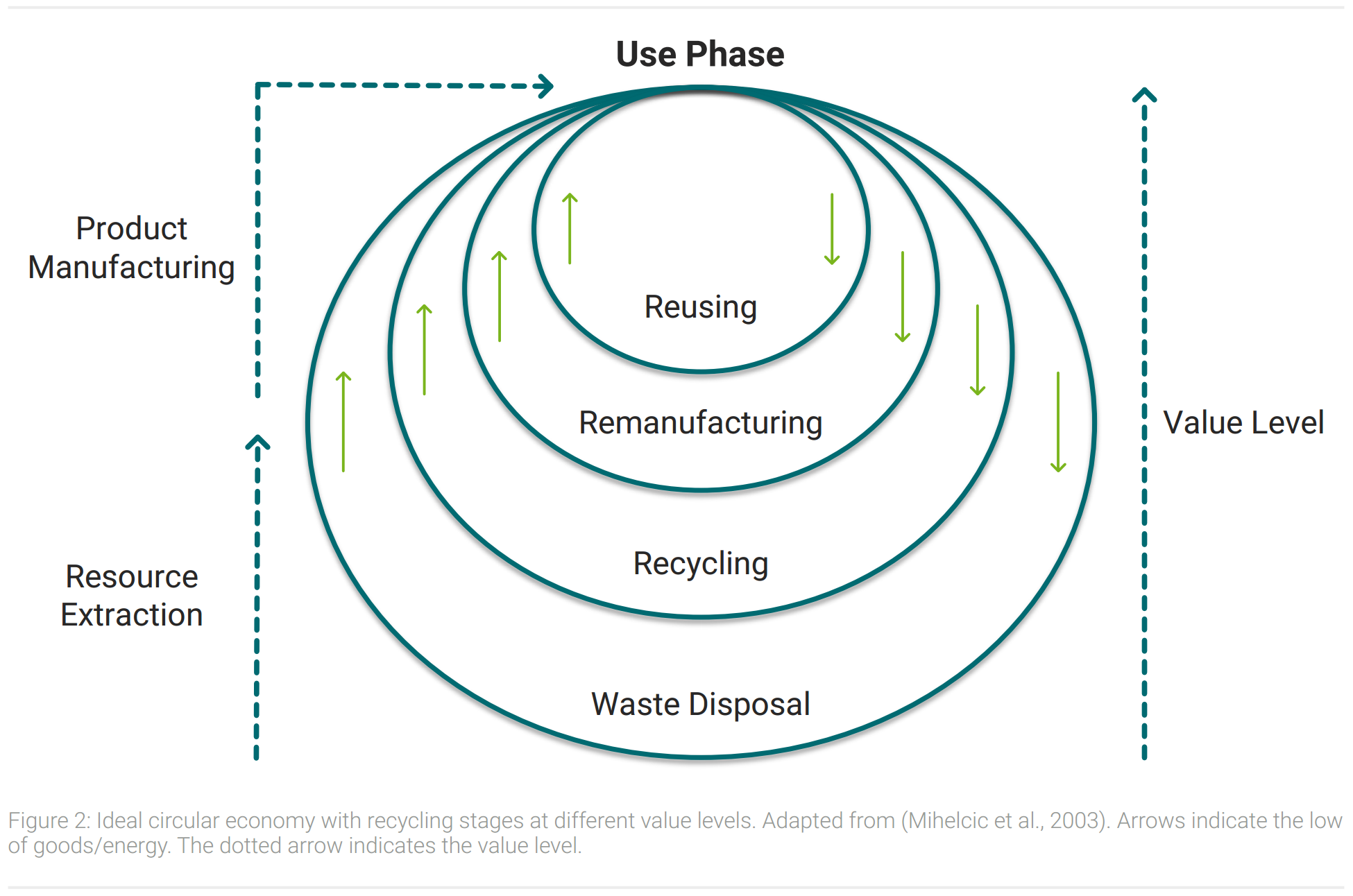
In contrast to today’s recycling circular economy prioritizes the value level. Products are supposed to be recycled on the highest value level possible. Figure 2 shows a schematic illustration of a value level focused circular economy.
All circles in figure 2 recycle products after their life cycle to be used again. Staying on a higher value level means that less energy and resources are needed to get the product back to its use phase (Korhonen et al., 2018). Consequently, a higher economic and ecological efficiency results in the prioritization of the inner circles of figure 2. Outer circles are sorted in descending order according to their value retention. Reusing products after their former lifecycle decreases the value level on an interim basis in a small extent. Remanufacturing products uses further resources and more energy. When products are recycled their raw materials are extracted and large quantities of resources and energy are consumed to reproduce products. Disposal of waste leads to a value level of zero, as remaining resources need to be extracted again and are not available in a usable form.
Circular economy has become more popular in the latest years as more politicians recognize the concept. An important module of the European Green Deal is the transition towards a circular economy for Europe (European Commission, 2019). This module is operationalized in the Circular Economy Action Plan of the European Commission, which describes measures to implement circular economy in European value chains (European Commission, 2020a).
2.4 CCU Technologies
There are numerous possibilities to use CO2 in the chemical industry or adjacent areas. In this chapter only a few applications that have a high impact today or in the future will be described.
2.4.1 Synthesis Gas
Synthesis gas is an important basic product in the chemical industry. The mixture of hydrogen and carbon monoxide can be used to produce a wide range of chemical products and synthetic fuels. Because of the numerous secondary products synthesis gas has a high production volume. Hence, producing synthesis gas with a CCU application could cause high emission reductions (Foit et al., 2017).
The H2/CO mixture can be synthesized directly from CO2 and H2O via high temperature co-electrolysis. The process combines CO2 and H2 electrolysis. The reduction of CO2 is slower than the reduction of H2O. Using the reversed water-gas shift left over CO2 can be converted to CO. As two different processes are used, it is possible to adjust the H2/CO ratio by changing the reaction parameters (Foit et al., 2017). The co-electrolysis works with solid oxide electrolysis cells and solid proton conducting electrolysis cells. The latter has the special feature that small amounts of methane are generated in the electrolysis process (Ebbesen et al., 2014).
2.4.2 Urea
Using CO2 in the production of urea is the standard process today. Ammonia and carbon dioxide are converted to ammonium carbamate under high pressure. The resulting ammonium carbamate produces urea via a dehydration reaction. The process is advantageous for the chemical industry because CO2 and NH3 react exothermic. Even though the dehydration reaction of ammonium carbamate is endothermic, the total process remains exothermic (Meessen, 2010). So, chemical companies do not need high energy inputs to produce urea.
Coupling the urea production with the production of ammonia has the advantage that both ammonia and CO2 that accrues in the production chain prior to the synthesis of ammonia can be used to generate urea. Today the process with the three steps, steam reforming, ammonia synthesis and urea synthesis remains a CO2 source. In the future hydrogen for the synthesis of ammonia could be generated in electrolysis facilities without CO2 generation. CO2 for the urea synthesis could come from other sources, making the process a CO2 sink (Driver et al., 2019).
2.4.3 Organic carbonates
Producing organic carbonates via CO2 is interesting as the greenhouse gas can be bound in products and in addition toxic reactants like phosgene and carbon monoxide can be substituted. There are two ways to produce organic carbonates using CO2 . Diols or two mono alcohols and CO2 can react to corresponding cyclic or acyclic carbonates. The reaction can be used to produce numerous simple cyclic and acyclic carbonates without metal-based catalysts (Lim et al., 2014). The reaction could become more important in the future, as electrolyte components like ethylene carbonate and dimethyl carbonate for lithium ion batteries can be produced with a lower carbon footprint (Xu, 2014). Epoxides or oxetanes and CO2 can be converted to cyclic carbonates or polycarbonates using metal-based catalysts. The reaction can convert terminal and some internal epoxides and oxetanes (Martín et al., 2015).
Polyether carbonate polyols can be synthesized in a similar way by increasing the stochiometric ratio of epoxides to CO2. Other than that, the reaction parameters and catalysts have to be adjusted. The exact product is dependent on the starter alcohol (Langanke et al., 2014). This process has been developed by Covestro. A demonstration plant already uses the process to produce “Cardyon” (Covestro AG, 2020). A mass fraction of 20 % of CO2 is the upper limit but this share already reduces the emission of greenhouse gases of the production of polyether carbonate polyols by up to 19 % (Assen & Bardow, 2014).
2.4.4 Methanol
Methanol could become very important in the energy and chemical industry. It could function as platform chemical for both sectors. Methanol can be used as energy storage or in the transport sector as fuel. Furthermore, Methanol is a basic chemical that is used to produce other high-volume chemicals like acetic acid and formaldehyde (Nyári et al., 2020). Even alkenes like ethene and propene can be generated from methanol (Chang, 1984). The high number of downstream products results in a high CO2 reduction potential, if Methanol is produced under incorporation of CO2.
Hydrogen and carbon dioxide can directly be converted into methanol and water. But the yield is lower than the conventional synthesis of methanol via synthesis gas (Inui, 2002). New catalysts might help to reach higher and more economic yields. So, the research focuses on new catalyst combinations and process technologies (Centi & Perathoner, 2014). Carbon Recycling International already uses this technique to produce 4000 t of methanol per year (Carbon Recycling International, 2021).
With regard to coming volatilities in the power grid due to the higher share of regenerative electricity generation it could be important to store energy. Excess electric power can be converted to H2 via water electrolysis. But H2 is difficult to store and transport. Synthesizing methanol from H2 and CO2 could provide a versatile energy carrier, as it is easy to store and transport. The needed CO2 could be captured locally by Direct Air Capture facilities (Fasihi et al., 2019). Furthermore, as stated above Methanol can be used in numerous applications (Weimer et al., 1996). This could also relieve the problem of the geographic inequality of the production of regenerative energies.
2.4.5 Synthetic fuels
Fuels with a reduced carbon footprint that are usable in conventional combustion engines could be important for the transition time towards fully electric transport systems. Synthetic fuels produced by the Fischer-Tropsch process meet this requirement. The synthesis provides a broad range of hydrocarbons by reacting CO with H2. Main products of the process are olefins and paraffins besides water as side product, but oxygenated hydrocarbons are also generated. Selectivity and conversion rates towards certain products can be controlled by the catalysts and reaction parameters used (van der Laan & Beenackers, 1999). Coupled with the process to produce synthesis gas from CO2 and H2 the Fischer-Tropsch process can generate synthetic diesel or kerosene in a sustainable way. CO2 could directly be used in the mentioned process via the reversed water-gas shift (Choi et al., 2017).
3 Method
To expand the scientific knowledge about CCU with the knowledge of the chemical industry expert interviews were used. The interviews were structured by a guideline with main questions, so every interview followed the same scheme. The guideline was designed to be open for detailed questions and expert specific questions. This approach allowed to generate usable information without large excurses. Additional information could also be generated using this approach (Gläser & Laudel, 2010). The following catalog of criteria has been used to find qualified experts in the field of investigation. Experts did not have to fulfill all of the criteria mentioned in the catalog, as this is partly impossible.
- Knowledge about CCU
- Knowledge about sustainability
- Knowledge about regulatory affairs
- Deep knowledge about specific companies
- Broad knowledge of the chemical industry
- A suitable position in the organization
- Diversified points of view
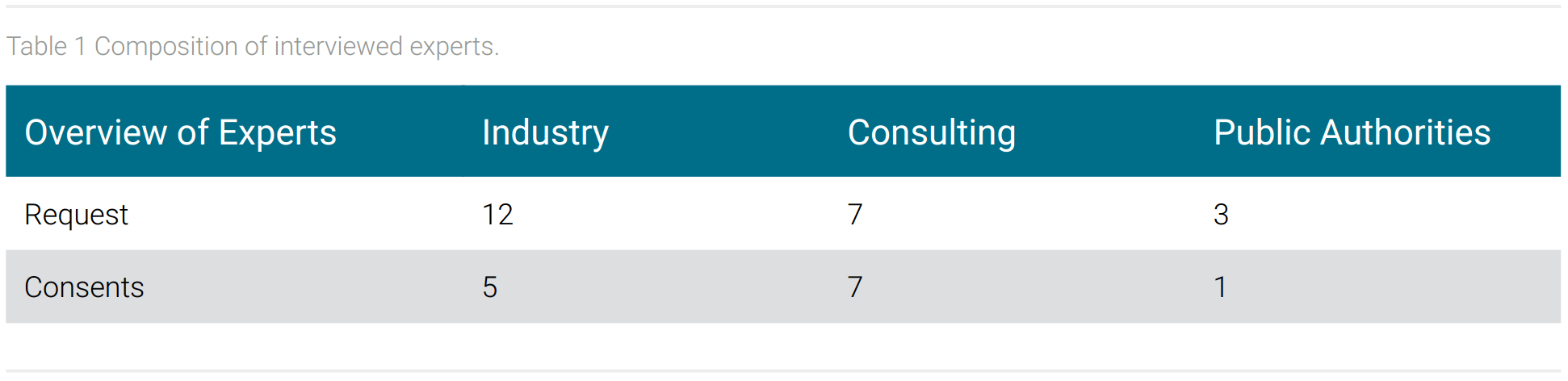
Of the 23 experts requested, 13 accepted and participated in an interview. It was possible to conduct interviews with experts from all three requested areas: industry, consulting and public authorities. The lowest proportion of experts came from public authorities. The composition of the experts is shown in table 1.
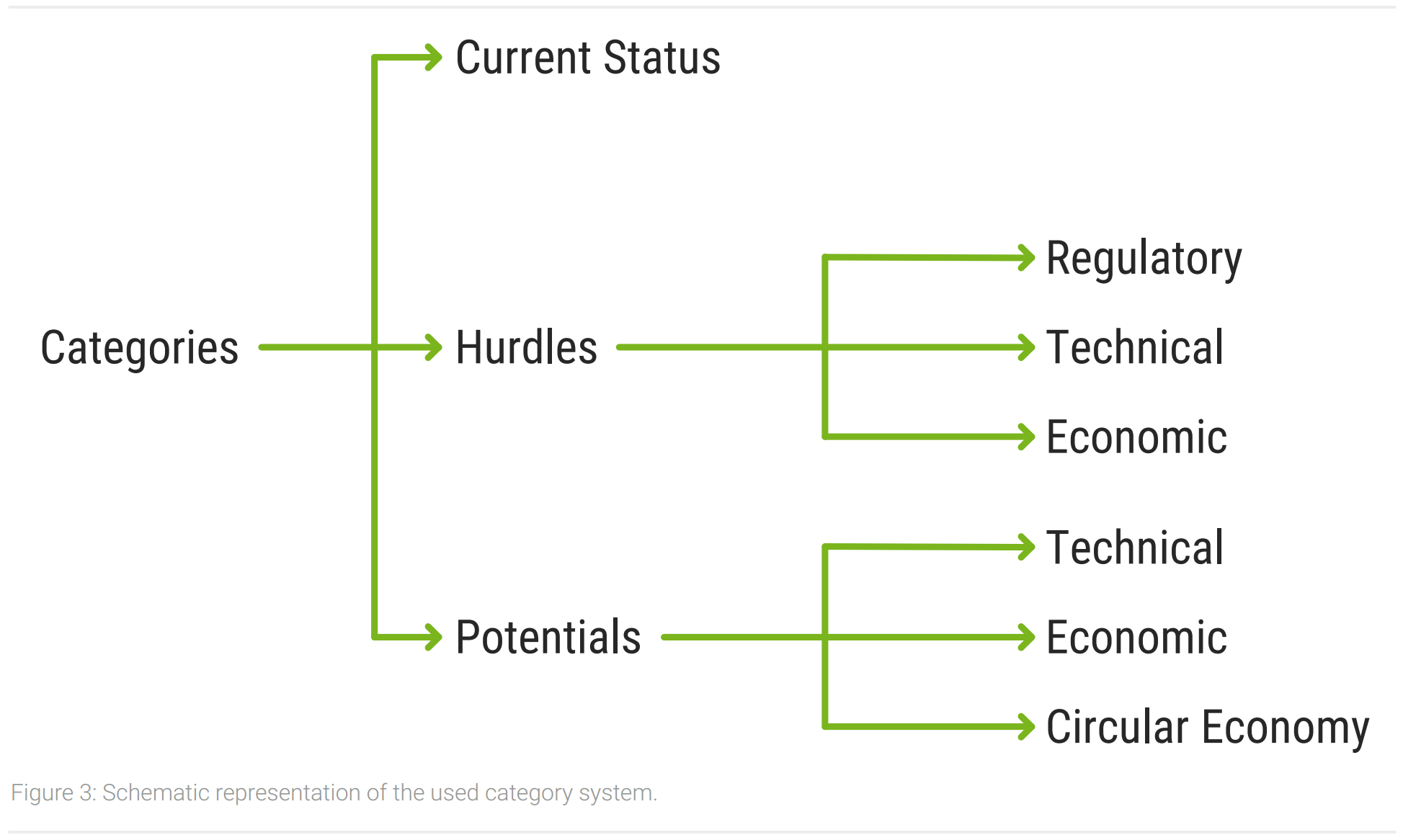
The interviews have been recorded and transcribed verbatim to avoid losses of information. A qualitative content analysis has been used to analyze the transcripts and derive results. This is particularly suitable for the open conceptualization of investigations and the identification of essential areas (Mayring, 2015). For an easier analysis the software MAXQDA has been used (MAXQDA, 2021). A category system has been constructed to extract information from the transcripts. Following the general conceptualization of the analysis, the category system has been designed in an open manner. This allowed to include aspects that have not been anticipated (Gläser & Laudel, 2010). A schematic representation of the category system is shown in figure 3. After analyzing and aggregating similar aspects, the information was structured and compiled to form the results in the next chapter. The subsequent discussion contextualizes the results of the analysis and the findings from the literature.
4 Results
4.1 Current status of CCU
Using CO2 as a raw material in the chemical industry has so far been based on the idea of using it where it seems energetically viable. Products that are higher in energy terms are obtained from energy-rich raw materials such as natural gas and crude oil. The new idea of CCU is to replace these raw materials and include more energetically expensive processes. With the rising priority of climate change discussions, such projects have received government funding in Germany. A well-known product of these subsidies is “Cardyon”, which is produced by Covestro. The synthesis process of “Cardyon” involves high-energy reactants besides the CO2. Therefore, the use of CO2 to improve the environmental assessment of the process lends itself. In addition to the energetically overloaded synthesis pathway, the prospect of a margin bonus through the “green” production of premium products is also a driver for the implementation of CCU processes.
The majority of CCU processes, for example the production of highly discussed products such as fuels or plastics, are not commercially viable yet. On the one hand, this is due to the high amounts of energy required to get the inert CO2 molecule to react. For the carbon footprint of the products to actually be improved by the CCU approach, only renewable electricity must be used. On the other hand, CCU processes are at the beginning of their development and the efficiency can still be significantly increased. Compared to conventional fuels and petroleum-based chemicals, whose production processes have been improved over decades, CCU processes are still at an early stage. Thus, the efficiency of the processes is difficult to compare so far.
4.2 Regulatory Hurdles for CCU
4.2.1 Political perspective
Due to societal pressure, political boards have become strong drivers of climate protection. They initiate large funding programs for projects in the field of sustainability and quotas regulate recycling and the use of fuel additives. Thus, political boards are also a driver for the development of CO2 utilization processes. Accordingly, developments in this area depend on politics and its decisions.
To get large companies to invest in CCU technologies a clear direction must be set by politics. An example is the handling of CCS. If large amounts of CO2 are stored by CCS in the future and the economy continues to be based on fossil fuels, then alternative processes such as CCU will have a weak economic position. Similarly, the required duration for CO2 to be bound in products is a concern. The supposed advantage of CCS is that it can sequester CO2 indefinitely, whereas most CCU processes can only store CO2 for a certain duration, from weeks to a few decades. For example, if a sequestration period of several years is required, then the use of synthetic fuels in transport, especially in aviation, is not feasible.
Recently, there have been many serious changes in the political framework in the energy sector, but also in related areas. These short-term changes within one or a few legislative periods make it difficult to calculate the profitability of plants that will only pay off after decades. The political framework must be more stable so that companies can calculate with a certain degree of planning reliability. Investments in CCU technologies must be viable for at least 30 years. The risk for companies to invest in such a technology and the required plants would otherwise be too high. This means that politicians must set up conditions that will last beyond a few legislative periods.
4.2.2 Deductibility of CCU in the EU ETS
The EU ETS is the central instrument in Europe for the mitigation of climate change. Storage of CO2 in the sense of CCS is deductible in the EU ETS, so no certificates have to be handed in for stored CO2. Besides one specific exception, this deductibility does not apply for CCU. Although CO2 can be captured with these processes and, depending on the product type, is not emitted for a long time, companies are not able to take advantage of this cost benefit.
Deductibility of CCU in the EU ETS would enable the greatest CO2 reduction potential of CCU. By transferring CO2 from other sectors to chemical companies, the potentially bound amount of CO2 increases enormously. However, without deductibility, no company would pursue this effort, as it would not be an economic advantage. Power, cement and steel plants, but also other industrial facilities, will continue to emit CO2 for decades. Especially in developing and emerging countries, the economy is expected to decarbonize more slowly than in the EU. By using CCU the emissions of these industries could be bound in products and a contribution to climate protection could be made before the decarbonization of these industries is finished.
4.2.3 Methodological basics
In order to make the use of CO2 more transparent and set up the deductibility of CCU in the EU ETS, better methodological basics for the accounting of CO2 emissions and bindings are needed. There are already problems with possible cases of double charging through the EU-ETS and national CO2 trading systems. The accounting problems would be exacerbated by CCU, where the boundaries between emissions, reductions and avoidance can quickly become blurred. The following example will exemplify the problem.
If CO2 from a cement plant is converted to synthetic fuels by the chemical industry, to whom is this CO2 reduction credited? How high is the reduction of CO2 emissions compared to conventional fuel? Theoretically, the CO2 reduction would be exactly 50%, due to the substitution of fossil fuels. Consequently, who pays for the EU ETS allowances for the other 50 % of CO2?
These questions highlight the challenges of implementing CCU in the regulatory context. They need to be clarified through life cycle assessments and implemented in legal frameworks to provide certainty for companies and consumers. On the other hand, a clear regulatory framework in this area is also important for states, as CCU together with sector coupling and globalized companies could open loopholes to remove CO2 from a company’s balance sheet, even though it is actually emitted.
4.3 Technical Hurdles for CCU
4.3.1 Efficiency and Effectivity
An important technological hurdle is the low efficiency of CCU processes. This depends on the level of technological maturity and the associated scaling effects. The efficiency is expected to increase with a growing plant fleet and experience effects.
However, some processes such as the co-electrolysis also have process-related drawbacks. Most conventional chemical processes operate in a solvent or gaseous state. This makes it much easier to create scaling effects with conventional processes. Electrolysis processes are bound to the surface of the electrodes. Increasing the surface area of a system is more challenging and thus more costly than its volume. This is also true for water electrolysis, which is an important prerequisite for many CCU processes.
Another issue is the effectivity of CCU. CCU can presumably help to save CO2 emissions and substitute fossil raw materials. However, the question arises to what extent this is possible. If the example above of synthetic fuel based on CO2 from a cement plant is used again, then the theoretical reduction of the total CO2 emissions would be 50 %. This is because no fossil oil is needed to produce the fuel. In practical comparison, the reduction would be less than 50 %, because the transport and conversion processes have to be included. In addition, there is the important question of whether a 50 % reduction is sufficient to meet climate goals. As things stand, a 50 % reduction will not be sufficient. Thus, it is questionable whether more long-term binding methods like CCU based chemicals can withstand the requirements to achieve the climate targets.
4.3.2 Balance of Energy
An inherent problem of CCU is the energy balance of most processes. Efficient chemical processes are preferably designed to move from a high-energy feedstock to a lower-energy product, to reduce the amount of input energy required. Now, CO2 is a very stable and low-energy molecule. In the case of CCU, a low-energy molecule is used and higher-energy products are produced. It thus contradicts an energetically efficient chemical process. Most of the chemical products can be generated with moderate energy input using CCU processes. However, the production of synthetic fuels poses an even greater problem. These materials are used in particular because of their high energy density and represent a significant market potential for CCU. The desired energy density for the production of synthetic fuels from CO2 must be supplied by large amounts of renewable energy. This problem, which arises directly from consideration of the desired reactants and products, is one of the main drawbacks of CCU and will not be solvable due to its intrinsic nature.
4.3.3 Electricity and Hydrogen Demand
The decarbonization of the economy is accompanied by its electrification. Electrifying the entire economy will require large amounts of “green” electricity, which is not yet available. Renewable energy expansion is accelerating, but current expansion targets cannot keep up with the economy’s electrification goals. Thus, renewable energy could become the bottleneck of the economic transformation.
Manufacturing chemical and petrochemical products with CCU processes would require large amounts of “green” electricity. On the other hand, for climate protection reasons, it also makes sense not to use renewable electricity in CCU processes with high priority. This is because other measures to reduce CO2 emissions, such as the use of heat pumps or the electrification of transport vehicles, more efficiently convert the electricity used into CO2 reductions.
Hydrogen is often needed to manufacture products through CCU. However, it will also reach a greater demand in the future in other sectors of the economy, such as the steel industry. The supply of “green” hydrogen is likely to lag behind demand in the next few years. Thus, CCU processes with a high hydrogen demand, such as synthetic fuel synthesis or co-electrolysis, are in strong competition for this raw material.
4.3.4 Carbon Capture
CO2 capture from point sources is the only economic option to avoid CO2 emissions through carbon capture in the short and medium term. Contrary to the common belief that CO2 is a waste material and therefore free, carbon capture is energy intensive and expensive. In addition, depending on the flue gas captured and the desired synthesis pathway, capture is complex because impurities and trace substances have to be filtered out. The concentration of impurities in the exhaust gas of waste incineration plants, which could serve as a suitable CO2 source in the long term, is particularly high. Thus, not only the technical maturity of CO2 utilization needs to make great progress, but also the preceding process for raw material extraction for CCU.
While Direct Air Capture is an ideal solution for today’s economy and would significantly improve the long-term environmental assessment of CCU technologies, the concentration of CO2 in the atmosphere must be considered. The concentration of 0.04 % CO2 in the atmosphere is hardly sufficient for technical purposes. Even though there are initial pilot projects, the capture of CO2 from ambient air on a large scale is not yet economically viable. The volume flows alone, which would have to be moved to capture several tons of CO2 per day, would be enormous. This would further increase the energy input of CCU processes. Coupled with the general scarcity of renewable energy described above, it is more economical to tap point sources while they still exist.
4.4 Economic Hurdles for CCU
4.4.1 Costs
As the chapter on CO2 capture already shows, the raw material CO2 is an expensive basic product. Compared to crude oil, which is a high energy raw material, the price is not competitive. This increases the cost of all CCU processes. Depending on the origin of the CO2, costs can vary widely, but cheap sources such as natural gas production sites are not in the spirit of the circular economy and contradict the principle behind CCU.
In addition, CCU processes require the addition of energy, which crude oil or natural gas already provide. This means that large amounts of energy are required. These energy quantities must come from renewable sources as described above, otherwise one of the core ideas behind CCU is invalidated. As the demand for “green” electricity increases in the future, the price is also likely to rise. Thus, operational costs of CCU will not decrease in the short and medium term.
This is a particular problem for the economic viability of CCU, as operational costs make up a large part of total costs. That is due to the target size of production facilities for CCU products. In order to utilize economies of scale as efficiently as possible, very large plants are planned. As a result, the investment costs are high, but in comparison, the operating costs are disproportionately higher due to the high scaling of the plants. Hence they dominate investment decisions of participating companies.
4.4.2 Entrepreneurial Risk
Companies constantly have to take certain risks in order to develop innovations. As the following aspects show, the risks surrounding CCU they are very high. So many chemical companies do not invest in the new technology. There is little planning security, which makes companies tend to act conservatively. This is because there have hardly been any economic success stories in this area to date. There are also foreseeable organizational problems, as new supplier relationships will have to be created. While there have been hardly any relationships between industrial sectors in the past, CCU would have to rely on these intersectoral supply networks. For example, steel plants would have to become suppliers for chemical plants. However, there is a risk that these steel plants or other suppliers will be decarbonized in the long term and thus no longer be able to act as suppliers.
On the other hand, CCU is very dependent on the political perspective. As long as CCU is in competition with the use of fossil raw materials, real economic viability cannot be achieved. It can only be achieved through government levies or quotas. As a result, companies are more strongly influenced in their decisions by the government than in other technologies and business areas.
4.4.3 Margin
The costs addressed above have a direct impact on the achievable margins of CCU products. A significant proportion of the population is interested in sustainably produced goods and is slowly becoming more willing to pay for these products. However, the higher prices are not enough to absorb the increase in production costs due to CCU. This is because these higher margins have largely been achieved by companies at the end of the value chain. These companies still claim low purchasing costs for their products from the upstream value chain. Since the chemical industry rarely sells directly to the end consumer, but mostly bears the additional costs for the use of CCU processes, the margins at this point in the value chain are lower.
4.5 Technical Potentials of CCU
4.5.1 Technical Maturity
The current technical level of CCU is not yet high enough to build large industrial plants, but some demonstration plants for selected processes are already in operation. Since many CCU processes have already been tested in an industrial environment, the number of demonstration plants can be increased significantly in the next few years. By 2030, many demonstration phases could be completed and the construction of industrial plants could start. Due to the role model function of pioneer companies, like Covestro, the overall development speed could increase and more companies could start the implementation of CCU technologies. This is also due to the feasibility of CO2 utilization, because there are no scientific rules opposing it. One example is the production of synthetic fuels via Fischer-Tropsch synthesis. The principle has been known for a long time, but it has only recently been tested for large-scale production and applied industrially.
The rapid development of adjacent technologies such as water electrolysis is another positive aspect for CCU. This is because most CCU processes can only be performed with large amounts of “green” hydrogen. Due to the rapid development, the high hydrogen demand of CCU could be covered more easily in the future.
4.5.2 Large field of application
A variety of possible applications of CCU have already been shown. Modern chemistry makes it possible to use CO2 in various processes and to produce diverse chemical end products. This dimension of possibilities opens up great potential for CCU, as it makes the technology interesting for many companies. From basic chemistry to specialty chemistry and in the pharmaceutical and cosmetics industry, CCU can be used in almost all areas of organic chemistry. In the construction industry, mineral carbonation can also be used to bind CO2 and improve the performance of materials.
While classical chemical conversion still accounts for the majority of CCU processes, more and more electrochemical processes are also being developed to utilize CO2. In addition, biotechnological processes are being researched in the CCU sphere, like the Rheticus project by Evonik and Siemens. This project uses bacteria to produce butanol and hexanol. The project is particularly interesting because C4 and C6 molecules can be produced directly from CO2. Overall, CCU processes can be used to produce almost all relevant products in the organic chemical sector via various intermediate stages.
4.5.3 Platform chemicals
The wide range of applications of CCU is mainly due to the possibility of sustainable production of basic materials such as methanol, but also other important basic products such as alcohols and carboxylic acids. Since large parts of today’s chemistry are built up from these basic chemicals, the CO2 footprints of downstream products are also reduced.
Methanol in particular could be used as a platform chemical in the future, which can be produced directly from CO2 and H2 and serve as a basis for a variety of chemical end products. This is due to the chemical structure which can easily be converted into various products and the possible scalability of the production process of methanol. Since methanol can be used directly in fuels as a blending component or converted via some intermediate steps to polyoxymethylene dimethyl ether, which serves as an additive for diesel, the large automotive market can also be addressed.
Furthermore, methanol could be used as a chemical energy storage, so that surplus renewable energy is converted into methanol on site and can be stored and transported more easily. This would be a great advantage for remote locations that can efficiently produce solar or wind power. Methanol can be easily converted back into electricity during times of high energy demand.
4.6 Economic Potentials of CCU
4.6.1 Scaling effects
An important factor in reducing the costs and increasing the economic viability of CCU is scaling. So far, the largest projects are still in the demonstration phase and industrially relevant quantities are not being produced yet. The processes studied are scalable, because companies examine the scalability before investing in a technology. Only by using high scaling effects plants can reach production costs that are competitive in the international competition. As soon as the first industrial plants are built in the future, production costs will also fall due to natural economies of scale. Due to technical drawbacks mentioned above, this effect could be lower for electrolysis processes than generally assumed. Nevertheless, the costs of this type of CCU process will also decrease due to scaling. In addition, there are experience effects that increase the efficiency of a process the more it is used. To date, this effect is very small for CCU technologies. But in the future it could contribute to cost reductions.
4.6.2 Increasing margins
Companies that use CCU processes can exploit premium products or sustainably labeled products to economically serve the market. In the case of premium products, the price increase due to the use of CCU is less significant for the overall price of the product. Therefore, consumers are not discouraged to buy the product as much. This can be explained using a car as an example. If the conventional car costs 100 % and all the plastics in the car account for 5 % of the cost, then doubling the manufacturing cost of the CCU-based plastics would only result in a total price of 105 % for the car. This means that a price premium would be lower in proportion and demand for the car would fall less. This effect could be exploited with many premium products that are close to the customer or products that have a low material cost share in the total costs.
Higher margins can also be achieved with sustainably labeled products. Today many customers are willing to pay a price premium for “green” products. So far, this purchase behavior has been limited mainly to certain customer groups, but the customer base appears to be growing in the next years. As more and more companies recognize this potential, the range of products that could be marked with such a “green” label is growing. This perspective is particularly interesting for companies at the end of the value chain, but the aspect is slowly penetrating higher levels of the value chain as well. This is motivated by the increasing awareness of sustainability along the entire value chain and by companies demanding better product carbon footprints from their suppliers. In addition, margins for “green” products could rise sharply in the short and medium term due to increasing demand, for example if public procurement procedures are linked to sustainability criteria. Demand could then grow rapidly, while the supply can only rise up slowly due to newly built plants.
4.6.3 Prices
CO2 taxes and levies will rise continuously in the coming years. In the EU, the expected price increase in the EU ETS, even if CCU is not counted in the system so far, will lead to an increase in the price of fossil-based alternative processes. CCU will thus benefit indirectly. With the foreseeable levy increasing and other coming governmental subsidies, many CCU processes could become economically competitive with their conventional alternative processes in a relatively short time. Some projects are already close to an economic break-even and could be operating on an industrial scale before 2030. The cost increase of conventional processes could then lead to more companies turning to alternative processes.
If CCU becomes deductible through allowances in the EU ETS in the next few years, the industrial implementation of CCU could make a leap forward. Because companies would receive money for processing the raw material CO2 through CCU. In addition, chemical companies could convert their own CO2 emissions into products and thus reduce their costs. Also, CO2 from other industries could be used and a market for CO2 could be emerge. This would make CO2 a commodity with a financial value. The deductibility of CCU in the EU ETS would significantly increase the profitability of many CCU applications and could encourage companies to invest in this area. With a price of 50-100 € per ton of CO2 in the EU ETS, which is expected in the coming decades, many CCU processes would be economically viable and could be rolled out more quickly due to greater interest by investors.
4.7 Contribution to the Circular Economy
There will be a carbon demand in the chemical industry in the future, even if the society acts more sustainably and tries to reduce the use of corresponding products. If fossil raw materials are no longer allowed to be used as a carbon source in the future, only three major sources will be available to meet the industry’s demand for carbon.
There is the recycling of plastics, where a distinction must be made between mechanical and chemical recycling. The former is highly efficient in terms of energy, since plastics can be reused without requiring a high amount of energy. Chemical recycling is on the verge of a major breakthrough and the processes developed are very promising. It will lead to a reduction in the amount of residual waste incinerated. Both recycling methods can open up usable carbonaceous waste streams for the chemical industry.
The second source is biomass. Apart from the processing operations, the use of biomass to manufacture products is always carbon neutral and therefore attractive. Even high-energy biofuels can be obtained from energy-rich bioproducts such as vegetable oil or sugar. However, the industrial use of plants always comes with the disadvantage that these agricultural areas cannot be used for food. This problem will increase with a rising world population und climate change. In addition, food production is likely to be prioritized over industrial use of biomass, so the latter may be curtailed in the future.
CCU represents a third option to meet the demand for carbon. In theory, CCU could supply carbon to the entire chemical industry. This would mean a significant additional effort for the industry, since all products would have to be built up from a C1 molecule, but in modern chemistry it would be possible with just a few exceptions. In terms of quantity, there should also be sufficient CO2 available. At least in the medium term it is unconceivable to assume that the major CO2 emitters such as power plants and industrial plants will be electrified worldwide. If there is a long-term shortage of efficient CO2 point sources, capture of CO2 from ambient air could be used. CCU with Direct Air Capture as a CO2 source would also create a fully closed carbon cycle within the chemical industry. However, since this technique is more inefficient than using point sources, it would only be used if point sources were not available. Or if this separation method is required by customers.
Practically, CCU will probably not have to meet all the demand for carbon on its own. Nevertheless, CCU can bridge the gap between the supply through mechanical and chemical recycling, the bioeconomy and the demand of the chemical industry. Thus, CCU can contribute to a fossil-free economy. In the chemical industry, the use of CO2 could have a double positive effect because it can produce its own products from CO2, which are thus managed in a circular way. On the other hand, by using CO2 from external point sources, it can also help other sectors to advance the circular economy and contribute to climate protection. In many sectors, this could only be a transitional solution, for example in the steel sector, which will switch to direct reduction with hydrogen in the long term. In other industries, which have unavoidable CO2 emissions, this industrial symbiosis could be of a long-term nature. So, CCU results in a high contribution to the circular economy for the chemical industry.
5 Discussion
With regard to regulatory hurdles, the recognition of CCU in the EU ETS presents itself as one of the main problems. This problem could be addressed by the EU in the near future, as the EU’s Circular Economy Action Plan will also establish a regulatory framework to certify the removal of CO2 from the atmosphere. However, the extent of deductibility of CCU in the EU ETS in the sense of the chemical industry is still an open question. It must be taken into account that there are other CO2 reducing technologies that are more effective for climate protection and are therefore given higher priority by policymakers. Examples include the installation of heat pumps in buildings and electromobility. Thus, it may be that CCU is deliberately promoted less to steer companies directly towards these alternative technologies. Nevertheless, there is a political will to focus on sustainable technologies in the EU in the long term. So regulatory hurdles in this sector could be reduced in the future.
The big problem from a technical and economic perspective is the efficiency of CCU processes. Even the most sophisticated technology will not be able to change the energy level of CO2. However, modern catalysts can significantly simplify the conversion of CO2 in terms of energy and thus improve the overall energy balance. The efficiency of CCU with regard to the use of renewable energy and also hydrogen will probably be the biggest issue in the coming years. This is because many industrial sectors will require these resources and the supply can only grow at a limited rate. For example, the chemical industry alone could use as much renewable electricity after the electrification as Germany currently produces in total.
Companies investing in CCU today are taking a risk by doing so, as they will have to invest a lot of research work and money in the processes before they can be used commercially. In addition, the processes are highly dependent on the regulatory framework. Therefore, it is difficult for many companies to approve investments in CCU processes. This high entrepreneurial risk can be reduced by some support measures, but the currently high and uncompetitive costs remain for the time being. In addition to the risks of the technology itself, new supplier relationships for CO2 from point sources will need to be formed. However, chemical companies must accept the risk if they want to be among the pioneers and thus among the winners of the technology if it becomes permanently established. Tesla is a good example for an early adoption of a sustainable technology and demonstrates the economic advantage of such investments. The development of CCU processes, which are not profitable today, could therefore be seen as an investment in the future.
The scalability of CCU is an important criterion for the cost degression of the technology. This relates to both the production processes and the sales markets. The production of methanol by CCU could play an important role, since this market is already large and the process is known and easily scalable. The advantage of fuels based on methanol or other synthetic fuels is that they can be used without major modifications to conventional systems. This applies to cars, but also to aircrafts and ships. Particularly in the area of long-distance transport, i.e. in the area of aircraft, ships and also trucks, these fuels have great potential due to their high energy density. A chemical economy based on methanol is also a possible future scenario and could significantly increase the market potential of sustainably produced methanol.
Undeniably, CCU is a way to establish a circular economy for the chemical industry. It can be built up in cooperation with other industries by using point sources or within a closed loop by capturing CO2 from ambient air. Since the first alternative would provide a transitional solution for many industries and is more efficient, it should be prioritized. In the long term, a switch could be made to Direct Air Capture once point sources are decarbonized. The advantage of this circular economy for the chemical industry is not only the sustainable and climate-friendly production of its products, but also the increasing independence from oil and gas imports. Decoupling from fossil resources and using sustainable processes could also improve the image of the chemical industry in the long term.
The use of CO2 as a raw material can reduce the emissions of the chemical industry and even emissions of other industries. In contrast to many other emission reduction options, it is possible to create value in the process. However, the absolute reduction potential strongly depends on the products manufactured and there are many other technologies, that have a higher CO2 reduction potential per amount of renewable energy used. Nevertheless, CCU is becoming an important technology on the way to a carbon-neutral economy. In certain sectors, carbon-containing products will be used forever and a complete mechanical and chemical recycling will not be possible. To meet the residual carbon demand, only CCU remains. The bioeconomy will probably only be able to contribute a small part due to population growth and the reduction of agricultural land due to climate change. Moreover, unlike almost all other approaches, CCU can recapture CO2 already emitted while contributing to value creation. This will likely be necessary in the future due to already high concentrations of CO2 in the atmosphere that have to be reduced to an acceptable level. So CCU will not be the universal solution. But the technology can make an important contribution to climate protection and the circular economy.
References
Assen, N. von der, & Bardow, A. (2014): Life cycle assessment of polyols for polyurethane production using CO2 as feedstock: insights from an industrial case study. Green Chemistry, 16(6), pp. 3272–3280.
Assen, N. von der, Jung, J., & Bardow, A. (2013): Life-cycle assessment of carbon dioxide capture and utilization: Avoiding the pitfalls. Energy & Environmental Science, 6(9), pp. 2721-2734.
Baena-Moreno, F. M., Rodríguez-Galán, M., Vega, F., Alonso-Fariñas, B., Vilches Arenas, L. F., & Navarrete, B. (2019): Carbon capture and utilization technologies: a literature review and recent advances. Energy Sources, Part a: Recovery, Utilization, and Environmental Effects, 41(12), pp. 1403–1433.
Carbon Recycling International. (2021, March 11): Projects: Emissions-to-Liquids Technology. https://www.carbonrecycling.is/projects#project-goplant
Centi, G., & Perathoner, S. (Eds.). (2014): Green carbon dioxide: Advances in CO2 utilization. Wiley.
Chang, C. D. (1984): Methanol Conversion to Light Olefins. Catalysis Reviews, 26(3-4), pp. 323–345.
Choi, Y. H., Jang, Y. J., Park, H., Kim, W. Y., Lee, Y. H., Choi, S. H., & Lee, J. S. (2017): Carbon dioxide Fischer-Tropsch synthesis: A new path to carbon-neutral fuels. Applied Catalysis B: Environmental, 202, pp. 605–610.
Covestro AG. (2020, December 24): CO2 als Rohstoff. https://www.covestro.com/de/sustainability/lighthouse-projects/CO2-dreams
Driver, J. G., Owen, R. E., Makanyire, T., Lake, J. A., McGregor, J., & Styring, P. (2019): Blue Urea: Fertilizer With Reduced Environmental Impact. Frontiers in Energy Research, 7, Article 88.
Ebbesen, S. D., Jensen, S. H., Hauch, A., & Mogensen, M. B. (2014): High temperature electrolysis in alkaline cells, solid proton conducting cells, and solid oxide cells. Chemical Reviews, 114(21), pp. 10697–10734.
Ember. (2021, June 6): Carbon Price Viewer. https://ember-climate.org/data/carbon-price-viewer/
European Chemical Industry Council. (2019): 2020 Facts & Figures of the European chemical Industry. European Chemical Industry Council.
Determining transitional Union-wide rules for harmonised free allocation of emission allowances pursuant to Article 10a of Directive 2003/87/EC of the European Parliament and of the Council, December 19, 2018.
On the monitoring and reporting of greenhouse gas emissions pursuant to Directive 2003/87/EC of the European Parliament and of the Council and amending Commission Regulation (EU) No 601/2012, December 19, 2018.
European Commission. (2019, December 11): The European Green Deal: Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions. Brüssel. European Commission.
European Commission. (2020a): Circular Economy Action Plan: For a cleaner and more competitive Europe.
European Commission. (2020b, September 17): Stepping up Europe’s 2030 climate ambition: Investing in a climate-neutral future for the benefit of our people. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committe of the Regions.
Establishing a scheme for greenhouse gas emission allowance trading within the Community and amending Council Directive 96/61/EC, October 13, 2003.
Amending Directive 2003/87/EC so as to improve and extend the greenhouse gas emission allowance trading scheme of the Community, April 23, 2009.
On the geological storage of carbon dioxide and amending Council Directive 85/337/EEC, European Parliament and Council Directives 2000/60/EC, 2001/80/EC, 2004/35/EC, 2006/12/EC, 2008/1/EC and Regulation (EC) No 1013/2006, April 23, 2009.
Amending Directive 2003/87/EC to enhance cost-effective emission reductions and low-carbon investments, and Decision (EU) 2015/1814, March 14, 2018.
Fasihi, M., Efimova, O., & Breyer, C. (2019): Techno-economic assessment of CO2 direct air capture plants. Journal of Cleaner Production, 224, pp. 957–980.
Foit, S. R., Vinke, I. C., de Haart, L. G. J., & Eichel, R.-A. (2017): Power-to-Syngas – eine Schlüsseltechnologie für die Umstellung des Energiesystems? Angewandte Chemie, 129(20), pp. 5488–5498.
Geres, R., Kohn, A., Lenz, S. C., Ausfelder, F., Bazzanella, A., & Möller, A. (September 2019): Roadmap Chemie 2050: Auf dem Weg zu einer treibhausgasneutralen chemischen Industrie in Deutschland.
Gibbins, J., & Chalmers, H. (2008): Carbon capture and storage. Energy Policy, 36(12), pp. 4317–4322.
Gläser, J., & Laudel, G. (2010): Experteninterviews und qualitative Inhaltsanalyse als Instrumente rekonstruierender Untersuchungen (3. Edition). VS Verlag.
Inui, T. (2002): Effective Conversion of CO2 to Valuable Compounds by Using Multifunctional Catalysts. In C. Song, A. F. Gaffney, & K. Fujimoto (Eds.), ACS Symposium Series. CO2 Conversion and Utilization (Vol. 809, pp. 130–152). American Chemical Society.
Kätelhön, A., Meys, R., Deutz, S., Suh, S., & Bardow, A. (2019): Climate change mitigation potential of carbon capture and utilization in the chemical industry. Proceedings of the National Academy of Sciences of the United States of America, 116(23), pp. 11187–11194.
Kaza, S., Yao, L., Bhada-Tata, P., van Woerden, F., lonkova, K., Morton, J., Poveda, R. A., Sarraf, M., Malkawi, F., Harinath, A. S., Banna, F., An, G., Imoto, H., & Levine, D.: What a waste 2.0: A global snapshot of solid waste management to 2050. Urban development series. Washington, DC, USA. World Bank.
Kirchherr, J., Reike, D., & Hekkert, M. (2017): Conceptualizing the circular economy: An analysis of 114 definitions. Resources, Conservation and Recycling, 127, pp. 221–232.
Korhonen, J., Honkasalo, A., & Seppälä, J. (2018): Circular Economy: The Concept and its Limitations. Ecological Economics, 143, pp. 37–46.
Langanke, J., Wolf, A., Hofmann, J., Böhm, K., Subhani, M. A., Müller, T. E., Leitner, W., & Gürtler, C. (2014): Carbon dioxide (CO2 ) as sustainable feedstock for polyurethane production. Green Chemistry, 16(4), pp. 1865–1870.
Lim, Y. N., Lee, C., & Jang, H.-Y. (2014): Metal-Free Synthesis of Cyclic and Acyclic Carbonates from CO2 and Alcohols. European Journal of Organic Chemistry, 2014(9), pp. 1823–1826.
Martín, C., Fiorani, G., & Kleij, A. W. (2015): Recent Advances in the Catalytic Preparation of Cyclic Organic Carbonates. ACS Catalysis, 5(2), pp. 1353–1370.
MAXQDA. (2021, February 17): Die #1 Software für Qualitative & Mixed-Methods-Forschung. https://www.maxqda.de/
Mayring, P. (2015): Qualitative Inhaltsanalyse: Grundlagen und Techniken (12. Edition). Beltz Verlag.
Meessen, J. H. (2010): Urea. In F. Ullmann (Ed.), Ullmann’s encyclopedia of industrial chemistry. Wiley.
Mihelcic, J. R., Crittenden, J. C., Small, M. J., Shonnard, D. R., Hokanson, D. R., Zhang, Q., Chen, H., Sorby, S. A., James, V. U., Sutherland, J. W., & Schnoor, J. L. (2003): Sustainability science and engineering: The emergence of a new metadiscipline. Environmental Science & Technology, 37(23), pp. 5314–5324.
Nyári, J., Magdeldin, M., Larmi, M., Järvinen, M., & Santasalo-Aarnio, A. (2020): Techno-economic barriers of an industrial-scale methanol CCU-plant. Journal of CO2 Utilization, 39, p. 101166.
OECD. (2019): Global Material Resources Outlook to 2060: Economic Drivers and Environmental Consequences. Paris.
Statistisches Bundesamt. (2021, June 6): Energy consumption in industry down 2.3% in 2018 on the previous year.
United Nations. (2015): Adoption of the Paris Agreement. United Nations Framework Convention on Climate Change.
United Nations. (2020, October 21): Sustainable Development Goals: Sustainable Consumption and Production.
van der Laan, G. P., & Beenackers, A. A. C. M. (1999): Kinetics and Selectivity of the Fischer–Tropsch Synthesis: A Literature Review. Catalysis Reviews, 41(3-4), pp. 255–318.
Weimer, T., Schaber, K., Specht, M., & Bandi, A. (1996): Methanol from atmospheric carbon dioxide: A liquid zero emission fuel for the future. Energy Conversion and Management, 37(6-8), pp. 1351–1356.
Xu, K. (2014): Electrolytes and interphases in Li-ion batteries and beyond. Chemical Reviews, 114(23), pp. 11503–11618.