Virtual Communities as an Organizational Mechanism for Embedding Knowledge in Drug Discovery: The Case of Chemical Biology Plattform
Abstract
In this paper, we document the lessons from the development of chemical biology platform in a major pharmaceutical company, and the outcomes of the early phases of this experiment. Although the concept of chemical biology is not new, its evolution and deployment in the drug development process is relatively new. The present experiment thus has to deal with both the scientific novelty of chemical biology, and organizational challenge of embedding it in the ongoing process of drug development. The notion of virtual communities or platforms overlaid on the traditional matrix of drug development served to introduce the approach, with some remarkable outcomes.
Introduction
In spite of widely heralded breakthroughs such as the human genome project, the innovation performance of the pharmaceutical industry as a whole has been lackluster. Although the specific figures are often under dispute, there is general agreement that the increasing costs of R&D1 coupled with a stagnating number of chemical entities reaching the market often are interpreted to be signals of declining innovation performance in the industry as a whole. This ‘innovation deficit’ is not due to the lack of diseases needing remedies, or of drug targets, upon which drugs can be designed (1). Rather, it is attributable to the process of drug innovation or the means by which targets are brought into the market.
No doubt recent years have witnessed remarkable innovations in the process of drug discovery and development. Advanced technological innovations have made it possible to do the screening of compounds for chemical properties at a high rate. But these innovations have not resulted in innovation efficiencies expected by their proponents; they have simply sped up our ability to screen compounds. The failures of these innovations call for rethinking the approach to the problem.
In this paper, we summarize a case study of the introduction of chemical biology (CB) platform to speed up the process and enhance the effectiveness of the drug discovery process in a major pharmaceutical company. CB platform deploys emerging ideas from knowledge management to distill lessons from past experiences in drug development; but unlike many KM approaches, CB approach opens up new scientific frontiers at the junctures of chemistry and biology, relevant to drug innovation.
The scheme of this paper is as follows. In the first section, we outline our view of KM as practiced in organizations. Our intent is not to be exhaustive in our treatment of KM, but highlight a few ideas to anchor our discussion of the development of CB platform. In the second section, we articulate the concept of CB to highlight the scientific novelty of this emerging field, as well as the unique features of this platform that set it apart from other KM exercises. Thus our treatment here is not to highlight the scientific aspects of this field, but the excitement, uncertainties and risks associated with its introduction. In the third section, we summarize the industry and organizational contexts that prompted the introduction of CB platform. In the fourth section, we discuss the introduction and preliminary outcomes of introduction of CB platform. Finally, we highlight the major lessons from the experiment.
I. Knowledge Management Approaches: An Overview
Knowledge Management (KM) emerged over the past five years or so as a significant management discipline with its own body of concepts, language, and practices (2). Broadly conceived, KM enables, supports, and encourages the following three interrelated foci:
1. The processes of discovering or creating new knowledge and refining existing knowledge;
2. The sharing of knowledge among individuals, and across all organizational boundaries; and,
3. The continued development and use of knowledge as part of individuals’ day-to-day work, and as part of decision-making.
But knowledge is not managed for its own sake. Rather the intent is to contribute to superior organizational performance (3), internal operating proficiency (4), and the quality-of-life of organizational members.
The evolving understanding of knowledge in organizational settings unavoidably brings in its wake two central knowledge challenges: (1) how to bring individuals together to create, share, and leverage knowledge, and (2) how to do so most efficiently and effectively in the interests of achieving the goals outlined above.
Two broad approaches to managing the knowledge challenges appear to dominate both the literature and practice in organizations: Organizations deploy human and organizational arrangements to practice KM; they also deploy information technologies to contribute to knowledge work. The organizational and IT approaches have been applied to both intra- organizational and inter-organizational locales (exchanges within and across the organization’s boundaries respectively). These two locales involve different contextual features that influence the content, direction, and intent of KM. For example, knowledge sharing– a central focus in much of intra organizational approaches– is influenced by the threat of intellectual property loss when applied to exchanges across the boundaries.
The organizational approach to KM explicitly addresses the human side of knowledge. It involves managing four interrelated elements so that individuals and groups better generate, share and leverage knowledge: 1) The choice, adoption and implementation of procedures or methods to bring individuals and groups together (who otherwise might not do so); 2) The formal and informal organizational settings in which individuals interact; 3) The organizational routines (e.g., process reviews, business case development) in which work occurs; and, 4) the organizational context in which all interactions and work take place, for example, creating and sustaining a knowledge-friendly culture (5,6,7).
The technological approach to KM involves the choice, adoption and implementation of information and related technologies. It requires the management of at least three distinct but related elements: 1) technologies that enable data gathering, massaging, mining and other data integration tools: these tools often involve establishing and refining many forms of data bases and/or automating, reconfiguring or integrating organization routines, processes or “best practices”; 2) technologies that enable data and information dissemination, distribution and deployment often requiring and enabling direct organization-wide or select involvement by individuals and groups; and 3) technologies that enable direct and real-time interactions among and between individuals and groups, often in distant
geographical quarters, so that they can converse with each other, share data and information, as well as offer opinions, judgments and critique (8,9). Its overarching intent is to enable the timely provision of more and higher quality data and information, both selectively and generally, to individuals and groups throughout the organization.
The technological approaches dominated the early stages of KM, and were useful in understanding the patterns within explicit knowledge as captured by electronic data bases. However these approaches could not migrate to scientific disciplines within organizations. Recently organizational approaches (e.g., communities of practice or COP’s) have been introduced as KM mechanism in scientific circles. However, in most of the current practice, these approaches were a means of transferring tacit knowledge from scientist to scientist. Several characteristics of chemical biology approach pose unique challenges to KM, a topic to which we now turn.
II. Chemical Biology
Two intertwined characteristics of CB set it apart from current KM applications. First, it is an interdisciplinary field that necessitates collaborations across disciplines; second, at present, it is in an embryonic stage that creates significant uncertainty about its definition, content and potential. We will take up each before we discuss their implications for drug development.
Interdisciplinary field
Historically, chemistry was primarily focused on structure and synthesis, and biology with function. Research into structure-function relationships remained an undeveloped interdisciplinary topic. In drug discovery both disciplines are important. As articulated by Wess, Urmann, & Sickenberger (2001), chemistry is necessary for the identification of new lead compounds, their optimization to clinical candidates, and for the provision of sufficient amounts of these substances for further studies and for development or scaling up. Biology’s need is transparent: After all, drug discovery is for treating biological malfunctions in the human body.
Over the years, the dialogue between chemists and biologists have been deepening, partly stimulated by the pressures of the pharmaceutical industry. Yet the chasm between the two remained, and many of the interdisciplinary aspects of the relationship between the two remained undeveloped. Recently, decoding the human genome has led to the estimate that out of more than 30,000 human genes, at least 1,000 are significantly involved in the emergence and course of disease (11). In turn, this has led to the conclusion that there might be 5000 to 10,000 genes that are targets for new drugs. These conclusions imply that the race is intensifying in the pharmaceutical industry as to who can develop the targets into commercially viable drugs. Furthermore, given these advances in biology, chemists now have a strong incentive to evolve their field as to remain relevant in today’s research context.
One approach to sketching the structure – function relationships is chemical biology (CB). ‘Chemical biology’ is a term arguably first advanced by Schreiber and Nicolau in a series of papers (12, 13). In a broad sense, CB aims to create biological response profiles by small molecules, selected on the basis of our state of knowledge about the structures and functions of biological targets. To accomplish this, however, biologists and chemists have to jointly generate knowledge about the structure and function of biological targets, and turn this knowledge into new molecules and then create relevant biological responses. Although the field is beginning to be established in the academia, it had not been implemented in the pharmaceutical industry.
Embryonic Field
Given the relatively recent emergence of CB, the scientific uncertainty surrounding the efficacy of this approach is unknown or at best uncertain. At this stage of development, CB promises rich dividends by concentrating research into structural and functional relationships. Patent remedies to address this problem are not yet available in the pharmaceutical industry. Rules need to be found for the design of profiles and a technology- integrated and information-based approach that transcends the synthetic skills and particular preferences of the chemists or the historic areas of activity of the firms needs to be followed.
The embryonic nature of the field is reflected in another set of circumstances. Currently, there are no individuals who are professionally trained and certified as chemical biologists; there are few universities which offer programs in this field. Thus for aspiring scientists, there are few role models of success: They will have to innovate and chart their own paths as they participate in the development of the field.
Implications for drug development
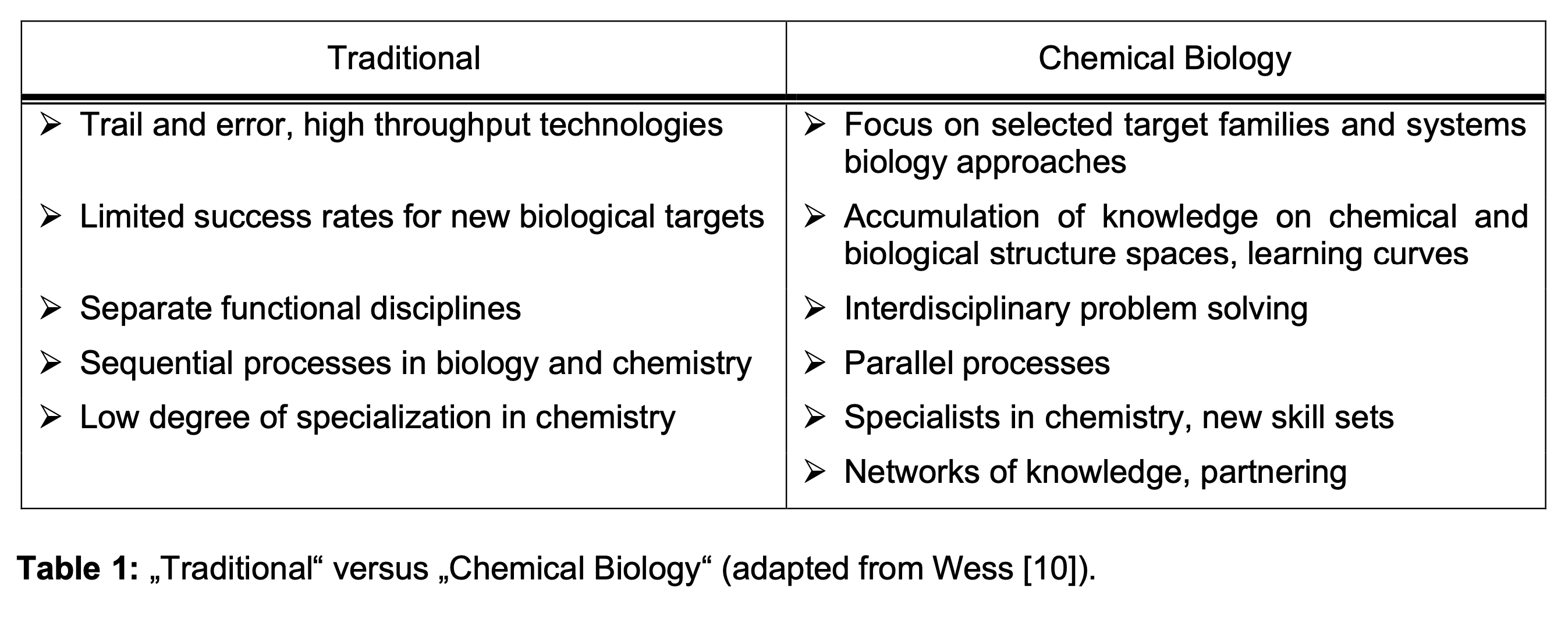
CB implies a radically different process of drug discovery. This approach was first articulated by Douglas in his 2000 keynote address at the Drug Discovery and Technology conference (14). See Table 1 for these differences. Especially in lead generation, the existing approach relies on trial and error method combined with high throughput technologies, sequential orientation and dominance of functional silos. CB requires a focus on targeted families and system biology approaches, interdisciplinary problem solving and parallel, information-driven and technology approaches.
These differences have two major implications:
- Since CB involves reorienting the process of drug discovery, any drug discovery and development organization that wants to institute CB will have to undertake a significant organizational change effort.
- CB requires building up scientific knowledge, a
process that can benefit from knowledge management (KM) approaches being instituted in organizations. Although KM has to date been employed in operations and management decision making, CB requires adaptation of these approaches to deal with the uncertainties of this embryonic field.
III. Industry and Organizational Context
Industry context
The pharmaceutical industry of the 21st century faces unparalleled challenges. Rising clinical trial costs due to difficulty in recruiting patients, expanding development programs, more chronic and degenerated diseases and longer development times have led to a condition where the innovation productivity of the industry – new medical entities relative to the dollar invested has declined. This perceived lack of productivity is worsened by the industry-wide realization we underscored above, brought home by the recent successes of the human genome project — that there are finite targets which all firms will be interested in their attempts to find cures for various diseases. These conditions, together with increasing societal expectations are putting pressure on pharmaceutical firms simultaneously to speed up and increase the effectiveness of the drug development process.
Various organizations have responded differently to these threats. But the organizational contexts of pharmaceutical firms display similarities.
Organizational context
As in a typical pharmaceutical firm, project teams are the units of innovation in Aventis. Various projects are managed by cross-functional teams. However, at Aventis, the Research and Development organization is made up of a matrix of globally coordinated as well as site specific functions. For example, chemistry, functional genomics, toxicology, clinical pharmacology are globally coordinated functions with units at each of the three major Discovery sites. Each discovery site has groups of Biologists (molecular biologists, biochemists, pharmacologists) that specialize in specific disease or therapeutic areas and form the core of the early stage cross functional teams. The role of the functions, be they globally coordinated or site-specific, is to supply the best people (knowledge) and technological solutions to address the specific challenges of the project team. These project teams drive drug discovery and development, with leaders expected to make decisions.
These teams operate in the Drug Innovation & Approval (DI & A) group within Aventis. DI&A is organized into functions and disease groups: Global functions cut across therapeutics areas, whereas therapeutic areas confined to a single site. Thus, individuals operate in a matrix, function or therapeutic area and project teams. Aventis renamed its R&D organization: Drug Innovation and Approval (DI&A) to emphasize the importance of its scientists focusing their activities on discovering innovative drugs and getting them approved. Unique to Aventis, DI&A organization is the Lead Optimization (LO) organization, consisting of the globally coordinated disciplines of Pharmacokinetics, Toxicology, Clinical Phase 1 and 11A. Lead Optimization bridges Discovery and late stage clinical Development, Phase11B and 111. The disciplines in LO support the project team in early testing whether a compound demonstrates the biochemical proof of concept and finally the clinical proof of concept, before the company commits the significant resources that are needed in late stage, Phase 11 and Phase 111 development.
In the past, Aventis scientists were project- focused: they did a project and moved on to the next. As a result there was no cross-project transfer of learning. Functions enable knowledge capture and transfer, but their focus is naturally functional excellence, albeit global. Site-specific disease groups similarly limit knowledge capture and deployment to their respective disease groups. The limited cross project transfer of knowledge led to a context where ‘targets’, the focus of CB, were typically not tracked.
IV. Introduction of CB Platforms in Drug Development
Early discussions
CB at Aventis was the first implementation in large pharma, and it did not happen overnight. Over a period of three to four years, prior to the introduction of CB platform, discussions among very senior R&D managers focused on the gaps in the then prevalent drug discovery approaches. By 2000, this group had arrived at the conclusion that the links between structural biology and chemistry remained a major gap, and the trial and error approach can be improved by the deepening the knowledge of ‘chemical biology.’ This conclusion was imbued with a sense of urgency when it became clear that Craig Venter and others had arrived at an incredible breakthrough in the human genome project. Douglas in one of two keynote addresses at the 2000 Drug Discovery Forum & Technology forum in Boston decided to commit Aventis to the application of chemical biology, as one way to take advantage of the potential classes of targets that were implied in the other keynote address that was presented by Craig Venter.
Management strategy
After the initial decision to commit to CB approach, the task of crafting a management strategy to implement this approach began. Although the senior leaders had a clear conceptionof what CB should do, the rank and file scientists who would actually be developing the scientific concepts, models and methods of CB were not privileged to be part of these early deliberations. An initial decision was to appoint a leader for the initiative. The chosen leader of the CB initiative was a long-term insider, a well respected scientist, with global experience, and strong interest in philosophy and innovation.
A first approach to sparking the interest of the scientists met with mixed results. A kick off meeting on CB with 25 promising Aventis scientists was held in Germany with the help of two McKenzie consultants in the fall of 2000. There was no real excitement and a lot of skepticism: Unlike the senior managers, they did not see much value in the new approach. The leader of the CB initiative initially considered the meeting to be a disaster: It was “scary” to hear such skepticism from young scientists.
Out of these early experiences, deliberations and other concurrent initiatives being implemented in the Aventis organization emerged a management strategy that was built upon three major anchors: (1) Incremental or pilot approach; (2) Project focused science; (3) The concept of virtual platform.
1. Incremental approach
This approach was a direct outgrowth of the experiences with other organizational changes that Douglas had introduced in some of the Aventis predecessor companies.. The skepticism of scientists convinced the senior R&D managers that rather than adopting an organization-wide approach, it may be necessary to adopt a slower, incremental approach to introduce and build the CB initiative on a pilot basis The pilot approach involved starting with a small group of scientists focused on a specific set of projects, and over time following up with several other projects. It was easier to locate a small number of enthusiastic scientists within the corporation, and their successes, both scientific and organizational, would ensure the interest of others.
Over the period of two years, Aventis launched four CB platforms: 1) Kinase, 2) G Protein Coupled Receptors (GPCRs), 3) Protease and 4) Ion channels and transporters.
2. Project-focused science
As we saw in the conception of CB, the scientific challenges involved were interdisciplinary lying in the intersection of chemistry and biology; thus, CB involved scientific work requiring removal of the basic/applied science divide. The focus was on rapid learning and knowledge development, through leveraging results of experiments on specific members of a class to determine applicability or reason for differences in results with other members in the class. Such knowledge should improve the predictability of finding good lead compounds for members of the target family (Kinase, GPCRs, Protease, or Ion channel and Transporter). The senior managers chose to focus this CB approach on the support of the work of ongoing project teams to ensure that ‘better compounds, faster’ are produced. This approach involved keeping the effectiveness of drug development at the center stage, and developing CB initiative as a means to enhance the business objective.
During the early stages, covered by the study, the CB platforms initiated focused on the performance of lead (compound) discovery and generation, the earlier stages of drug development. The expectation was that CB platforms should be able to demonstrate results in the short run with respect to the speed and efficacy of lead generation. The choices of the platform were guided by the extent to which they were likely to facilitate drug discovery. Indeed all the four platforms accounted for over 60 % of compounds produced within the company in the 4 years following their introduction. The initiatives that fell outside of Aventis’s main projects were not covered by this approach.
3. The concept of a virtual platform
As we noted earlier, like most pharmaceutical companies, Aventis R&D organization is a matrix: scientists belong to a function and to a disease group and to a project team. In this structure, functional excellence and knowledge of the disease are systematically brought to bear upon the drug discovery and development decisions. However, the knowledge of the target classes is ad hoc, and judgmental. Indeed, as we have noted earlier, the promise of CB is to infuse the drug discovery and development with the knowledge of the target classes, so that the process can be sped up and made more productive. This means a third dimension, over and above function or disease group and project, should be added to the prevailing matrix.
For the organizational form, Aventis settled upon the concept of virtual platform, an idea borrowed from the notion of communities of practices (COP) in Knowledge Management (KM). A virtual platform is a ‘collateral organization,’ made up of scientists with significant experience, working parallel to the existing drug discovery and development matrix. Several key characteristics of this virtual platform may be enumerated:
• Catalytic function: The function of a virtual platform is to influence through knowledge the discovery and development process. Since CB focuses on the linkage between structural biology and chemistry, it enables more rational decisions. A virtual platform is expected to infuse the project teams with knowledge about the targets pertinent to the challenges they are facing. This may result in speeding up of discovery or early termination of potentially infeasible compounds and targets.
• Target focused basic research: A CB platform is expected to create new insights regarding the linkages between chemistry and biology. This may involve distilling the experience of various projects regarding particular target classes, both within and outside the company to draw generalizations, including computer based modeling. Indeed, this research provides the necessary knowledge base to carry out the catalytic function.
• Internal organization: A virtual platform has a clear internal organizational structure. It has 1) a platform leader, and a core team consisting of several members, 2) a sponsor, who is a member of the senior management team that supports the leader and the core team, 3) several strategy groups, each led by a core team member working on specific scientific or science-related challenges, populated with individuals drawn from R&D organization as when and necessary. Each platform has significant operational autonomy, although all the platforms are encouraged to learn from one another. Thus, a virtual platform is not a team, but an organization with at least four levels. In theory, it interfaces with the project teams as and when necessary.
There are significant similarities between the virtual platform approach and prevalent KM approaches. The virtual platform is about knowledge capture and deployment; it uses IT to its advantage for both its operations (use of website) and codification of knowledge (e.g., creation of libraries and data bases); it resembles COP’s. There are important differences as well. First, the virtual platform concept focuses on generating new knowledge, not merely knowledge capture. Second, being project-focused, it cuts the delay between knowledge capture and deployment. Third, a virtual platform is significantly larger in size, sometimes resembling a small bio-tech firm.
Implementation
Over two years, Aventis implemented four platforms: Kinase, GPCR, protease and ion channels and transporters. As noted earlier, they were chosen for their potential contribution to the business purpose. For example, Kinase was chosen as the platform given its significance for two very important disease groups: oncology and immunology, and also due to the fact there was a large in house library of compounds making it an easy ‘demonstration project.’
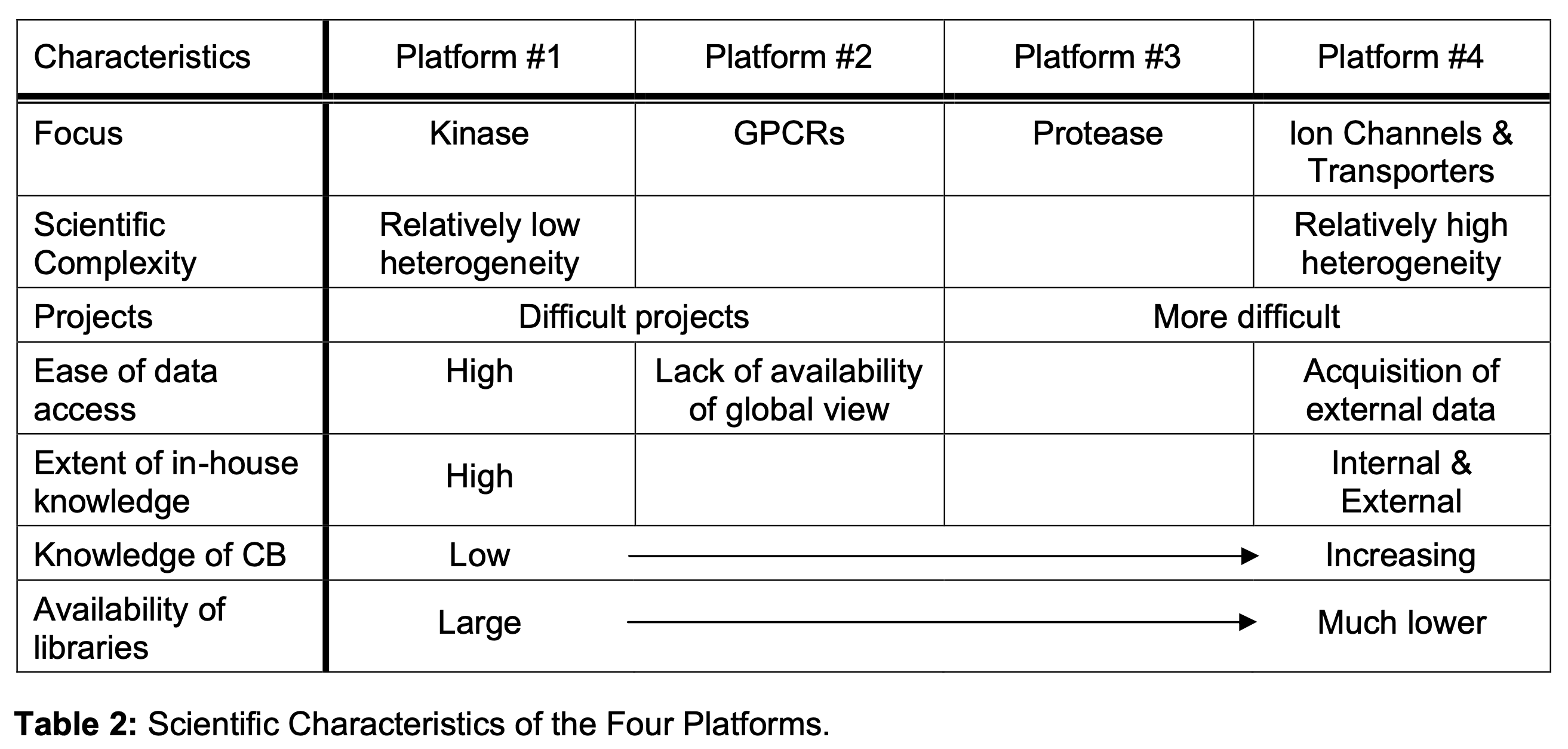
A comparative summary of the scientific characteristics of the four platforms is presented in Table 2. As shown in the table, the four platforms were oriented to different disease groups, and had different degrees of in house expertise to rely on. They were markedly different in terms of the key scientific challenges for several reasons:
- The targets themselves were different. For example, Kinase, unlike ion channels and transporters, represented a relatively homogenous group.
- The drug discovery projects addressed by the platforms differed in complexity.
- Internally, the accessibility of the data posed differing challenges to different platforms.Indeed these differences imply that the activities of various platforms would differ significantly. Similarly, a comparative analysis of the organi- zational characteristics of the four platforms is presented in Table 3. The table underscores several highlights:
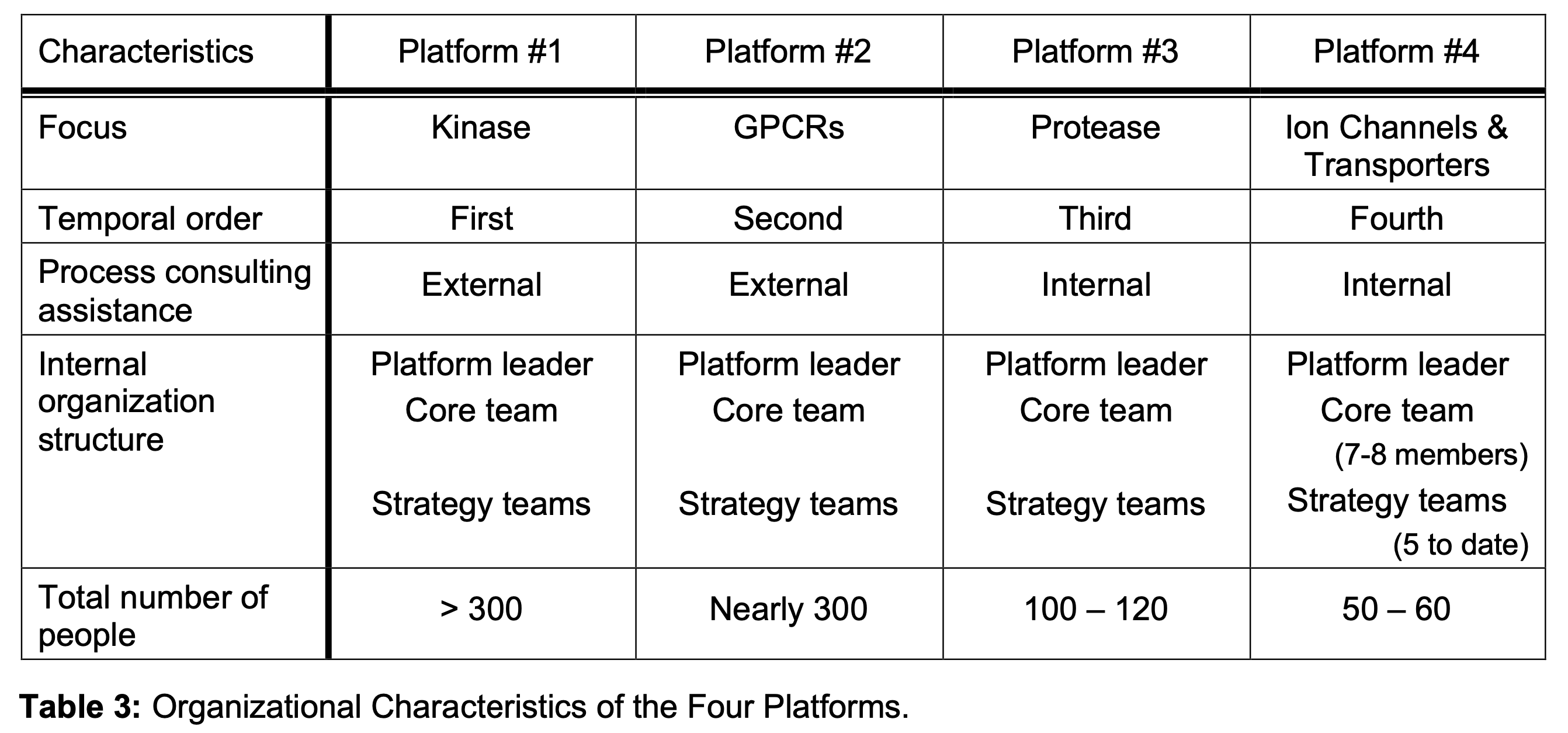
- There was a standard template for almost all the internal organization characteristics of the platforms. Of course, the platforms differed in their total size.
- The tasks confronting each platform and the adopted mode of operations differed from one another in significant ways. This reflected the differences in scientific challenges enumerated above.
- There was an attempt to learn from the earlier platforms. For example, in later platforms, internal consulting replaced external consulting. This was facilitated by a ‘Book of Knowledge ‘that captured experiences, problems and solutions as the Kinase and also later platforms were established. Similarly, later platforms, by intention, adopted a planned approach, unlike the earliest one, Kinase.
- Over time, interface with the project teams became stronger. The Kinase group members were reluctant to inject their knowledge into the working of project teams; this reluctance decreased over time, and was much less in ion channels and transporters.
- Technology alliances were common, which spanned the spectrum from setting up scientific advisory boards, to purchase of data bases, outsourcing of some activities
Indeed, one of the difficulties of assessing the accomplishments of a virtual platform, or for that matter any catalyst, is due to the fact that a platform’s influence is indirect, i.e., through the effectiveness of project teams’ decisions. Nevertheless, there is reason to believe that the platforms yielded a significant return on investment.
V. Key Lessons Learned
The introduction of CB platform in Aventis represented a two-dimensional revolution in the way a pharmaceutical company conducted its drug discovery process. On the one hand, it represented a scientific revolution, opening up an interdisciplinary field, Chemical Biology, hitherto a neglected approach to natural sciences. No doubt this revolution was made necessary by the failure of technology based approaches to drug discovery to deliver; it was also made necessary due to the competitive pressures triggered by the success of human genome project. On the other hand, it required a major organizational change within Aventis to accommodate the use of knowledge to enhance the effectiveness of drug discovery. Although any verdict on the long term effectiveness of CB platform will have to wait until the affected drugs are commercialized, the preliminary success of this approach emboldens us to suggest three key lessons from this experience: 1) strategic direction of a revolution; 2) Dynamic of management; and 3) Challenges of accountability.
- Strategic direction of a revolution: Revolution often does not occur in one leap. It sometimes comes through a series of baby steps. At the same time, the failure of the giant leaps may also serve as a prompt to rethink the approach to managing a revolution. Revolution involves both failures and successes. It is the management of failures or rather, framing them as occasions of learning that determines how quickly the revolution will spread. Finally, it requires in the beginning, a small band of committed individuals who are willing to share their experience, both successes and failures. Establishment of a living ‘Book of Knowledge ‘helps in the sharing of experiences. The key to managerial success is to be able to identify them early on and nurture them.
- Dynamic of management: Top down direction was essential. The direction took the form of the design of organizational mechanisms, not scientific approaches. Indeed the dynamic of management is essential: specifying the overarching goal, finding people, resources, and time in a matrixed organization, when most of these are not under the control of platform leaders.
- The challenges of accountability: Work approaches to various tasks differed across platforms, and their outputs were not standardizable. Also, measuring the effectiveness of catalytic function is tricky. We focused on examples not quantitative data. Thus, managers will not be able to hide behind numbers when forced to defend their decisions. At the same time, signaling to external scientific and financial communities is necessary to demonstrate the sense of accountability. Above all the contribution of the platforms was enhanced by having shared and aligned objectives between the core members of the platforms and the heads of functions, whose members were supporting project teams of relevance to the particular platform.
The approach to building the CB platform employed the concept of virtual platform, akin to the communities of practice which have dominated the organizational approach to KM. The virtual platform concept employed communities of scientists to examine the knowledge base; in that it was similar to COP’s. However, the virtual platform differed from COP’s prevalent in KM practice in several ways. First, the platform was built to create knowledge from an interdisciplinary group of scientists; second, there was relatively tight linkage between knowledge acquisition and utilization, and consequently more tangible results; by the utilization of technology ( including computer modeling), virtual platform interfaced organizational and technological approaches in KM. In this way, virtual platforms were an improvement over the COP’s. However, we do not know conditions under which COP’s and virtual platforms can be successfully introduced in organizations; this remains a major research opportunity for the future.
Acknowledgment
We thank Andrea Bazer, Bruce Barron, Peter Nestler and Heiner Glombik for the time spent with us on preparing this paper.
References
[1] Wess, G. (2002). How to escape the bottleneck of medicinal chemistry. Drug Discovery Today, Vol. 7, No.10, May 2002, 533-535.
[2] Narayanan, V.K. and L. Fahey (2002). An application of Rescherian Epistemology ot Knowledge Management: Implications for IS/It professionals. Paper presented at the Connecting Learning and Critique Conference held at Cambridge University, UK, July 2002.
[3] Hanson, M., Nohria, N. and Tierney, T. “What’s Your Strategy for Managing Knowledge?” The Knowledge Management Yearbook 2000-2001, Butterworth-Heinmann, Boston, MA, 2000.
[4] Drucker, P. “Knowledge-Worker Productivity: the Biggest Challenge,” The Knowledge Management Yearbook 2000-2001, Butterworth-Heinmann, Boston, MA, 2000.
[5] Brown, J.S., and Duguid, P. The Social Life of Information, Harvard Business School Press, Boston, MA. 2000.
[6] Leonard, D. and Sensiper, S. “The Role of Tacit Knowledge in Group Innovation,” California Management Review, (24:3), 1998, pp. 112-132.
[7] Spender, J.-C. “Making Knowledge the Basis of a Dynamic Theory of the Firm,” Strategic Management Journal, (17:2), 1996, pp. 45-62.
[8] Marwick, A. D. “Knowledge Management Technology,” IBM Systems Journal, (40:4), 2001, pp. 814-830.
[9] Ruggles, R.L. Knowledge Management Tools, Butterworth-Heinemann, Boston, 1997.
[10] Wess, G. Urmann, M. & Sickenberger, B. “Medicinal Chemistry: Challenges and Opportunities,” Angew. Chem. Int. Ed., 2001, Vol.40, No.18, 3341-3350.
[11] Drews, J. (2000). Drug discovery: A historical perspective. Science, 287, 1960-1964.
[12] Schreiber, S.L. & Nicolau, K.C. (1996) Chemical biology, Vol. 3, 1-2.
[13] Schreiber, S.L. & Nicolau, K.C. (1997) Chemical biology, Vol. 4, 1-2.
[14] Douglas,F.“DrugDiscoveryParadigmfor the New Millennium,” Keynote speech delivered to Drug Discovery Technology 2000, IBC’s Fifth Annual Congress, August 14-17, Boston, 2000.