Outsourcing of Pharmaceutical Manufacturing – A Strategic Partner Selection Process
The pharmaceutical industry is a growing industry, but companies struggle to capitalize on this growth because of a variety of challenges: shortening patent lives, strong pressure on prices, strict regulations, and the shifting of growth to emerging countries. Outsourcing of manufacturing is increasingly seen as a way to reduce operating costs and improve competitiveness. But external manufacturing is moving away from a purely opportunistic approach of transferring overcapacity to external partners or outsourcing of manufacturing to low-cost countries to reduce costs towards a more strategic approach, where external service providers are seen as partners. The ability to establish and manage strategic partnerships is seen as a key competence. This paper addresses this aspect and focuses on strategic partnerships to increase competitiveness of large pharmaceutical companies by outsourcing activities from chemical production through partly finished products to finished goods packaging. An action research approach was used based on a single case study of a global leading pharmaceutical company. A partner selection process consisting of seven consecutive steps, including the criteria for the partner selection, was developed for pharmaceutical companies with their highly regulated, quality focused manufacturing processes and history of vertically integrated production. It was also shown that, besides having the right process in place, the appropriate organizational structure has to be established.
1 Introduction
The outlook for the pharmaceutical industry is promising (Ernst & Young 2010; Price Waterhouse Coopers 2011). The 2009 market of $775 billion USDollar is expected to grow to over 1 trillion in 2014 with 6% annual growth. Probably the single most important driver in the pharmaceutical industry is time-to-market (Shah 2004). As a consequence, pharmaceutical companies have focused their skills on drug discovery, development and marketing. This is also reflected in the increasing numbers of scientific publications on management of R&D in the pharmaceutical industry (Piachaud 2002; Hess and Rothaermel 2011; Bianchi et al. 2011a; Bianchi et al. 2011b; Festel 2011; Schuhmacher et al. 2013). But although the industry is growing, major pharmaceutical companies struggle to capitalize on this growth, because they are challenged by a variety of trends (Shah 2004; Fujiwara 2013). There are shortening patent lives and even active patents provide lower barriers to entry, because there are many product alternatives in nearly all therapeutic areas: either alternative compounds (“me-too drugs”) or off-patent generics. The traditional blockbuster sales model is likely to disappear. There is strong price pressure for health expenditures, as those who pay for health care are exerting strong price pressure and influencing prescribing practices. This means, for example, that in order to be approved, new drugs must address new therapeutic areas or have very significant cost or health benefits as compared to existing treatments. These industry trends put pressure on the margins and have made it difficult for today’s companies to fight the competition in terms of profits. This suggests a need to find new and alternative ways of gaining a strategic and competitive advantage. One aspect is the significant changes in the area of supply chain management within the pharmaceutical industry. There is a general trend for companies to divest excess capacity resulting from having many local manufacturing sites, and to move towards a global supply chain management process. Whereas in the past the ability to deliver and reliability were important, today cost efficiency and flexibility are key factors for pharmaceutical companies (Verhasselt et al. 2012). Currently, the cost of logistics in the sector is relatively high (Song and Wang 2009; Kumar et al. 2009; Lowman et al. 2012), particularly due to supply chains often having been optimized in accordance with tax or transfer price concerns. One of the main challenges, once the product is on the market, is to ensure responsiveness to fluctuating customer needs. Developing and managing sources of supply is a challenging process especially for pharmaceutical companies with their quality focused manufacturing processes and history of vertically integrated production (Bhadoria and Rajpal 2011). Booth (1999), amongst others, states that there is a welcome move away from viewing the supply chain as merely having to deliver security of supply at minimum cost, to a recognition of its ability to generate additional value for the companies, if they choose the right partners. As the final responsibility for the product remains with the pharmaceutical company, it is crucial that the outsourced business is well controlled (Fields 2004). The importance of choosing the right contractor cannot be exaggerated, as some of the largest pharmaceutical recalls have been due to inadequate effort when selecting or monitoring contractors (Waggener 2003).
As research oriented companies concentrate on discovery and development activities, they rely more and more on external partners. One approach is to establish strategic partnerships in other areas. These are established for various reasons: to obtain access to knowledge and new technologies, to obtain access to new markets or expand global reach or for horizontal or vertical integration in the value chain (Zhang et al. 2013). The partners use synergy effects and combine their strengths to aim for growth and profit enhancement or improved cash flow. The ability to establish and manage strategic partnerships is seen as a key competence (Bath 2003). Based on a survey of US, UK, and continental European companies, Kakabadse and Kakabadse (2005) concluded that the best run companies of the future will focus more on establishing strategic relationships with a number of key business partners. The results strongly indicate that partnership alliances and performance driven contracts will become as important as the current preferred, trusted supplier relationship. But strategic partnerships raise questions concerning intellectual property ownership, technology transfer, hiring away of employees, splitting of profits and expenses, duration and termination of the relationship, risk of capital investments and many other business issues. The relationships are often complex as a result, and can be subject to extensive negotiation.
The decision making often does not follow a structured approach and is not pursued in a systematic way, or processes are just borrowed from other industries. As the product life cycle in the pharmaceutical industry is longer, more highly regulated and more complex than in other industries (Gu and Li 2010; Bhakoo et al. 2012; Citron 2012; Ren and Yeo 2006; Lee 2007), there is a need for a specific and customized partner selection process for this industry. But a surprisingly large number of pharmaceutical companies do not have defined processes for finding, choosing and managing contract manufacturers (Linna et al. 2008). Whereas there are several research papers focusing on partner selection processes in general (Ding et al. 2013; Lau and Wong 2001; Crispim and Pinho De Sousa 2009; Diestre and Rajagopalan 2012; Li et al. 2008; Zolghadri et al. 2011) or on the partner selection process in other technological fields (Ramani et al. 2001; Collins and Bechler 1999; Wittstruck and Teuteberg 2011), there is a lack of industry specific strategic partner selection processes for the pharmaceutical production processes in the academic literature (Chen and Hung 2010; Zhang et al. 2013).
To close this gap, this paper focuses on strategic outsourcing, i.e. the establishment of strategic partnerships for outsourcing manufacturing in the pharmaceutical industry. Strategic outsourcing is bringing in external service providers to manage essential tasks that would otherwise be managed by in-house personnel. In contrast to opportunistic outsourcing this means that this is done on a strategic level, i.e. to realize strategic goals of a company and not only as a tactical tool to use outsourcing on a short-term project basis to realize cost reduction potentials. This paper describes the selection of strategic partners for the manufacturing process, in particular within vertical partnerships of large pharmaceutical firms that have the manufacturing capacity but decide to outsource production for strategic reasons. Accordingly, the research questions addressed by this paper are as follows.
RQ 1: How can a strategic partner selection process for pharmaceutical manufacturing be defined and implemented?
RQ 2: What are criteria for the partner selection within such a process?
An action research approach was adopted to develop the partner selection process based on a single pharmaceutical manufacturing case study in collaboration with a pharmaceutical company belonging to the top ten global companies in this sector. The paper is structured in the following way. In section 2, the theoretical background regarding strategic partnerships and especially supply chain partnerships and outsourcing manufacturing in the context of the pharmaceutical industry is described. The research methodology is described in section 3. Section 4 provides the results and discussions, i.e. the partner selection process including the criteria for the partner selection. Finally, in section 5, the conclusions from the research presented in this paper in relation to the learnings from the analysis of the theoretical background are summarized and the outlook for future research is discussed.
2 Theoretical background
After describing types of collaborations and partnerships the role and importance of outsourcing in general are explained and outsourcing of manufacturing activities, especially in the pharmaceutical industry, is discussed.
2.1 Types of collaborations and partnerships
The types of collaboration can be classified according to various criteria. One option concerns the inter-business relationship – either vertical or horizontal co-operation. Horizontal co-operation is the most frequently used kind of collaboration, where companies collaborate with companies in the same value chain step and maximize the strengths of each company. Such co-operations can be found in partnerships where each partner brings its unique strengths to bear. In vertical co-operations, companies co-operate along the value chain. In the pharmaceutical industry, this form of cooperation can be found in joint efforts to develop and commercialize new products. In the past, pharmaceutical companies were characterized by a relatively high level of vertically integrated production (Bhadoria and Rajpal 2011). As the pace of change is increasing in many industries and product life cycles are shortening, the flexibility to establish partnerships according to business opportunities is becoming more and more important. This has led to the new company concept called virtual enterprise. This is defined as a temporary alliance of businesses that come together to share skills or core competencies and resources in order to better respond to business opportunities, and whose co-operation is supported by computer networks (Jung 2008). Byrne (1993) points out that this could even involve competitors in other fields that work together for a particular business opportunity to share costs and skills and to access one another’s markets. It will have neither central office nor organization chart, nor hierarchy, nor vertical integration. The virtual enterprise in the pharmaceutical industry is often characterized by a focus on project management to coordinate activities and the outsourcing of these activities necessary to achieve the project goal (Cavalla 2003; Boucher and Afsarmanesh 2013; Müller et al. 2013).
As manufacturing firms attempt to move up the value chain by offering additional services, service based manufacturing is an increasingly popular concept in literature (Neely 2008; Baines et al. 2009; Lay et al. 2010; Martinez et al. 2010; Wilkinson et al. 2009; Baines et al. 2009; Vandermerwe and Rada 1988; Smith et al. 2014; Zhen 2012) that often appears in context with virtual enterprises (Rodríguez Monroy and Vilana Arto 2010; Ducq et al. 2012; Sun et al. 2011). In a service based manufacturing scenario, the manufacturer supplier relationship does not follow a traditional customer supplier pattern, as the customer “asks for competencies rather than either only parts or only manufacturing capacity” (Urbani et al. 2002). According to Akbarzadeh and Pasek (2008) two different actors can often be distinguished. On the one hand there is the end user, who interacts with the market of finished goods as market supplier and whose core business is the interaction itself. The end user often adds value to the product through design, innovation, marketing, and branding. On the other hand there is the manufacturing service provider, who takes responsibility for the manufacturing response to the market and for customization. As a result, the core business of the manufacturing service provider, is manufacturing itself, which drives its focus on the necessary competencies and, consequently, leads to increased effectiveness. The concept of service based supply of manufacturing services was introduced by Urbani et al. (2002), who proposed manufacturing capacity supply as an extension of traditional outsourcing and an enabler for improved responsiveness and effectiveness. Schönsleben (2007) highlights the dynamic character of such partnerships in the area of supply chain management. He describes the transformation of a customer-supplier relationship into a strategic partnership in the supply chain according to the five characteristics quality, costs, delivery, flexibility, and co-operation in the logistics network.
There are also other concepts that do not focus on the transaction type but look at the overall system. One popular concept is to regard the industry as an ecosystem (Moore 1997; Isenmann et al. 2008; Isenmann and Hauff 2007) which represents an economic community supported by a foundation of interacting organizations and individuals – the organisms of the business world. The economic community produces goods and services of value to customers. The member organisms also include suppliers, lead producers, competitors, and other stakeholders. In general, the industry ecosystem concept is associated with a better economic and ecologic, due to a more efficient use of energy and materials. As such, the actors co-operate by using each other’s waste material, by-products and waste energy and in order to optimize the input of both raw material and energy and simultaneously reduce the output of waste emission (Li and Hao 2011; Chew et al. 2009; Maes et al. 2011; Geng et al. 2007). Over time, they co-evolve their capabilities and roles, and tend to align themselves with the directions set by one or more central companies (Côté and Hall 1995; Ritala et al. 2013). Within such an ecosystem, different types of company ecologies evolve. If the relationship between the organizations is cooperative and the strengths are complementary, this is a collaborative network.
2.2 Role and importance of outsourcing
All these modern types of collaborations and partnerships rely on outsourcing activities, i.e. covering of parts of the own value chain or supply chain by partners which are more suited to perform these activities. The main perceived advantages are reduction of costs and better allocation of resources in a project with variable demand, access to specific technology, expertise or skills either not present internally or less expensive/quicker than the internal alternative, greater flexibility, better management or spread of risk and freedom to concentrate on core functions. Jiang and Qureshi (2006) identified expected benefits of outsourcing and sort them into the following five categories: cost reduction, productivity growth, profitability increase, firm’s value improvement, and risk control. But outsourcing is not an optimal solution in all cases. It is a trade-off and involves some disadvantages, like loss of control (e.g. of quality and regulatory compliance) (Bath 2003; Linna et al. 2008), greater difficulty of co-ordination and management of external collaborations and contracts, less transparency (e.g. problems of evaluating and monitoring supplier performance), time taken to negotiate contracts, difficulties in agreeing on ownership or splitting of intellectual property rights, instability risks in case the external party becomes financially insolvent, merges or is acquired and generally dependent on the supplier.
Due to the broad array of potential engagement options, risk and benefits, there are many variations of outsourcing alternatives and several authors have attempted to develop a framework clarifying the wide spectrum of outsourcing arrangements, and their inherent risks and advantages (Sanders et al. 2007; Abdullah and Verner 2012; Sharp et al. 2011; Vitasek and Manrodt 2012; Braun et al. 2011; Hsiao et al. 2010b; Roy and Sivakumar 2012). Shared characteristics among early adopters of outsourcing have been shrinking product lifecycles and the growing need for agility and responsiveness to counterbalance increasing market volatility. As a result, fast moving industries, such as consumer goods manufacturing, like electronics and fashion, were more likely to embrace outsourcing when compared to slow-moving industries, like automotive and machinery. Increasing market volatility calls for new organizational forms enabling agility and responsiveness, which in turn forces firms to define and focus on their core competencies, streamline their operations, and leverage complementary competencies of suppliers to their competitive advantage in effectively managing continuous change (Akbarzadeh and Pasek 2008).
Outsourcing manufacturing is moving away from a purely opportunistic approach, transferring overcapacity to external partners or outsourcing of manufacturing to low-cost countries for the sake of cost reduction, towards a more strategic partnership approach. Han, Porterfield, and Li (2012) analyzed the impact of industry competition on contract manufacturing. This empirical study found that contract manufacturing is positively associated with supplier industry competition and the association is further moderated by focal industry competition and IT investment. One of few studies in this field based on financial metrices is the work of Plambeck and Taylor (2005). They studied profitability and investment in capacity and innovation in outsourcing manufacturing to contract manufacturers and concluded that contract manufacturing improves profitability for the industry as a whole only when companies are in a strong bargaining position vis-à-vis the contract manufacturer.
In the course of their literature review, Jiang and Qureshi (2006) determined that related outsourcing literature can be classified by three criteria: 1) outsourcing determinant, 2) outsourcing process, and 3) outsourcing result. The outsourcing determinant research studies the drivers behind a firm’s outsourcing decision, i.e. the “why” issues. Research on outsourcing determinants often refers to the transaction cost economics and the resource based view of the firm to study outsourcing agreements. The interest in the topic comes in waves and is strongly dependent on the status of the industry (i.e. maturity, business cycle, competition, regulation, etc.). The outsourcing process research focuses on outsourcing contract negotiation, partner selection, implementation, control, monitoring, and so on, i.e. the “how” issues. The process oriented research, most frequently concerns itself with outsourcing contract negotiation and partner auditing and monitoring (Jiang and Qureshi 2006; Mayer and Salomon 2006; Contractor et al. 2011; Ding et al. 2013; MacKerron et al. 2014; Zhang et al. 2013). Analysis of contract negotiations and bargaining power often uses the game theory model (Laiet al. 2009; Kumari et al. 2013; Vitasek et al. 2013; Leng et al. 2014).
The outsourcing result research studies what an outsourcing decision brings to the firm. Several researchers (Jiang and Qureshi 2006; Jiang et al. 2006; Hsiao et al. 2010a; Kitcher et al. 2012) see a gap regarding the third research area, the outsourcing result literature. Within the last decade, most academic studies have focused on understanding outsourcing decision determinants and outsourcing process control (Gilley et al. 2004). While contracting out is now broadly understood to be an attractive option, its specific impacts on firms’ performance and value, i.e. outsourcing results, have not yet been well confirmed by research. When researchers look to measure the financial impact of outsourcing results, they have usually been forced to rely on managers’ estimates rather than tangible metrics and ”much of the evidence that we have come across is anecdotal and case study oriented, and often based on non-financial metrics” (Jiang and Qureshi 2006). Jiang and Qureshi (2006) defined three main gaps in the outsourcing research literature: 1) lack of objective metrics for the evaluation of the outsourcing results, 2) lack of research on the relationship between outsourcing implementation and firms’ value, and 3) lack of research on the outsourcing contract itself.
2.3 Outsourcing in the pharmaceutical industry
A number of authors analyze and explore outsourcing in various industries and some of these papers cover the pharmaceutical industry. Strategic outsourcing has assumed an increasingly important role in the operations of established as well as emerging pharmaceutical companies (Getz 1997; Lowman et al. 2012). Specific advantages and disadvantages of outsourcing in this industry are explored, amongst others, by Cavalla (2003). Historically, most management attention has been paid to drug discovery and sales and marketing, the outer ends of the supply chain (Booth 1999). Therefore, in the pharmaceutical industry, research in the last few years has focused on R&D contractors and product development (Festel et al. 2010). Examples of such research are the work of Arranz and de Arroyabe (2008), which focuses on the choice of partners in the pharmaceutical industry for R&D co-operation, and Festel (2011) on outsourcing of chemical synthesis during the drug discovery phase. Another example is Piachaud’s analysis of the outsourcing of R&D by pharmaceutical companies to clinical research organizations which empirically analyzes the perceived advantages and disadvantages pharmaceutical firms have experienced (Piachaud 2002).
Whereas partnering in the drug discovery and development process as well as sales and distribution are well covered by many studies (Henderson and Cockburn 1994; Henderson and Cockburn 1996; Subramaniam and Dugar 2012; Macher and Boerner 2012), the outsourcing of pharmaceutical production is not. Methodologies are often just adopted from the manufacturing industry. Furthermore, research on partner selection, implementation, control and monitoring in the pharmaceutical industry in general and the production process in particular is rare, despite the fact that outsourcing the manufacturing of active ingredients, formulation as well as primary and secondary packaging is growing (Clinkscales and Geimer 2001; Linna et al. 2008; Ernst & Young 2010). Van Arnum (2006) estimates that in the US, the total value of commercial pharmaceutical manufacturing of finished dosage forms is 83 billion US-Dollar, of which 8-12 billion US-Dollar is outsourced. Manufacturing is often further differentiated into primary and secondary manufacturing (Shah 2004). The primary manufacturing site is responsible for the production of the active ingredients. This normally involves either several chemical synthesis and separation stages to build up the complex molecules involved, or fermentation and product recovery and purification in the case of biochemical processes. Secondary manufacturing is concerned with taking the active ingredient produced at the primary site and adding excipient inert materials along with further processing and packaging to produce the final products, usually in stock-keeping unit form.
An important area is the outsourcing of the production of active ingredients. Most pharmaceutical products involve primary active ingredient production (often multi-stage chemical synthesis or bioprocess) and secondary (formulation) production. Both of these stages are characterized by low manufacturing velocities and are hampered by the need for quality assurance activities at several points (Shah 2004). The oldest concept which has been broadly analyzed in the literature is contract manufacturing, which is considered as one category of outsourcing (Liston et al. 2007) and as such is often related to outsourcing topics. Contract manufacturing is regarded as a supply chain arrangement by which a manufacturing firm outsources some of its manufacturing processes to an outside supplier through a contractual agreement (Kim 2003). The pharmaceutical company maintains the ownership of the products while the contract manufacturer supplies labor and skills to manufacture the products. A more sophisticated example is the work of Naerhi and Nordstroem (2005), who analyze the challenges of choosing an appropriate contract manufacturing organization in the bio-pharmaceutical industry during the ramp-up phase for commercial manufacturing. This is a common scenario as the investments for bio-manufacturing facilities are high.
3 Methodology
After explaining why action research based on a single case study was chosen as research method, the details regarding data collection and analysis are described.
3.1 Research method
Action research has the dual goal of solving a problem and contributing to knowledge by participation of the researchers in the problem solving process (Westbrook 1995, Greenwood and Levin 1998). Therefore, action research is an appropriate method for developing a business process in a company (Eden and Huxham 1996, Coughlan and Coghlan 2002). This is achieved through a structured process with the steps 1) data gathering, 2) data feedback and analysis, 3) action planning and implementation as well as 4) evaluation (Susman and Evered 1978; Burns 2000; Coughlan and Coghlan 2002). Following the action research article by Pero and Rossi (2013), the desired outcomes of this research paper are the solution to the immediate problem and the lessons learned, but not to develop a new theory or to validate an existing theory.
Previous work on outsourcing topics has relied mostly on anecdotal evidence from case studies, surveys or other self-reported data to support assertions (Jiang and Qureshi 2006). Consequently, the action research approach in this article is based on a single case study of a globally leading pharmaceutical company in order to obtain in-depth insights into the subject. As suggested by Yin (2013) case studies are preferred for studying contemporary events where it is not necessary to control behavioural variables. A single case study approach is appropriate, if the aim of the research is to explore a previously unexplored phenomenon (Eisenhardt 1989; Eisenhardt and Graebner 2007; Yin 2013).
3.2 Data collection and analysis
In the action phase, one author was closely involved with the company in developing and customizing a company specific partner selection process including the criteria for the partner selection. The other authors served as sparring partners and supervisors to ensure that a systematic, structured and scientific approach was followed. The whole project was structured in the four phases 1) data gathering, 2) data feedback and analysis, 3) action planning and implementation as well as 4) evaluation.
- Data gathering: The information was primarily collected through direct interviews, direct observations and involvement in the company’s management activities. First, a relationship was established with the senior management of the company. Two of the authors were introduced to key people and, subsequently, embedded in the task force team responsible for the project. Semi-structured interviews (each interview lasted on average one and a half hours) with each of the key informants were performed. The interviews comprised a set of open questions to understand especially the supply chain management activities. Secondary data about the relevant companies, market and competitors were collected through documentary sources, such as annual reports, strategy plans, press releases on company web pages or through other forms of company reports and project documentation. Besides the objective to obtain an in-depth view about the situation, this information was also used to triangulate the data collected.
- Data feedback and analysis: The relevant data were continuously shared among the people involved in the project and frequently analyzed together in order to define clear objectives and to identify issues and needs as well as further areas for improvements. The confirmation of the results coming from the interviews was made through discussions within the team of authors and with the interview partners after writing down the interviews results. Contacts with contract manufacturers and suppliers were also established to discuss the results with them. Based on the concepts and trends identified during the literature research, which are described in the theoretical background section, the partner selection process described in the next section of this paper was developed within the task force team based on the interview results. Key statements regarding the partner selection process were extracted from the interview notes and consolidated based on the learnings from the literature review. The result was the description of a partner selection process with 7 steps. The whole process was then validated by all interview partners by making minor adjustments.
- Action planning and implementation: In cooperation with the involved managers, a plan for the implementation of the new partner selection process was defined. Answers were found to questions, such as, what type of change is required, which support and information are needed, and how the new partner selection process could work. The planned actions were then executed with the assistance of the two authors of this paper, who were members of the task force team.
- Evaluation: In order to generate generic knowledge on the partner selection process from this specific case, the results were verified and generalized within an evaluation phase by presentation and discussion with a group consisting of experts from four additional pharmaceutical companies (Eden and Huxham 1996, Greenwood and Levin 1998). Supported by quantitative as well as qualitative data, other perspectives had also been included in this verification process, such as those of outsourcing contractors and manufacturing service providers. The aim of these discussions was to obtain feedback from external experts regarding the partner selection process and to gain first insights whether this process could be also implemented in other pharmaceutical companies. Nevertheless, the aspects of generalizability and implementation in other companies are still open and should be part of further research as described in section 5.3.
4 Results and discussion
After describing the partner selection process developed within the research presented in this paper the criteria for the partner selection are explained.
4.1 Partner selection process
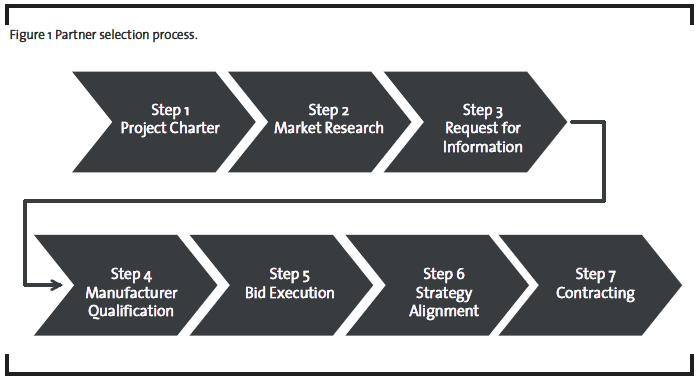
The importance of a professional partner selection process for pharmaceutical companies in the area of outsourcing manufacturing has been emphasized in the introduction and the theoretical background section. The partner selection process developed during the action research consists of nine consecutive steps and is illustrated in Figure 1. The preceding make-or-buy evaluation will not be further described in this paper and is a different field of research.
Step 1: Project Charter
The process starts with the development of the project charter. Either a company specific or a general project management template can be used. One widely used general project management methodology is the PMBOK Guide from the Project Management Institute. The key points of the project charter are the project scope, objective, participants, timeline, roles and responsibilities. Furthermore, the strategic intent of the project has to be specified, including milestones and assumptions. By that time, a preliminary business case including a profitability calculation is developed and internal or external competency screening has been done. The project charter is defined and agreed on by all stakeholders involved in the project.
Step 2: Market Research
A dedicated team is in charge of the search for and identification of potential strategic partners using market intelligence information and tools. Prematurely determining a preferred partner list based on a limited non-holistic approach should be avoided in this phase. It is important in this step to avoid personal preferences or selective interests influencing the selection. This could lead to high hidden costs in the end. If the internal resources are very limited and suitable tools are not available, a market scan can also be done using an independent external partner. The result of this step will be a long list of potential partners for external manufacturing. The phase may be time consuming but will deliver an important basis for decisions at the end.
Step 3: Request for Information
This step starts with the development of a specific request for information (RFI). In addition to the questions related to manufacturing capabilities for pharmaceuticals, the document includes background information about the objectives of the partnership, the RFI process timeline, deadlines and submission instructions, and a confidentiality agreement. In addition to those items, compliance with the code of conduct or a specific supplier code of conduct can already be included in the RFI. Preceding work has shown (Oehmen et al. 2010) that reference to a supplier code of conduct in an early phase of negotiation helps to mitigate risks related to production, for example workplace safety issues around hazardous materials. The dedicated sourcing team is responsible for releasing the RFI to the potential contract manufacturers identified, communicating and clarifying requirements, acting as the single point of contact for questions, ensuring on-time submissions, and providing feedback. This step is important for the clarification of the needs, as many questions are likely to be asked by the potential partners. It is important that questions arising during the RFI are clarified with all suppliers involved to establish an equal level of information for all participants. Therefore, these information updates during the process should be defined by a change control procedure and proactively managed by the sourcing team. After RFI submissions are collected from potential contract manufacturers, the team reviews them and comes to a shortlist of three to four companies. This selection is again very important and needs to be performed using a comparison matrix agreed with all internal stakeholders. Finally, all participants (selected and excluded) are officially informed about the results and feedback is provided, which is often appreciated and facilitates future RFIs.
Step 4: Manufacturer Qualification
The selected potential partners will then enter into the next phase where they are assessed by the technical assessment team following predefined criteria. The particular categories may be assessed by different experts, but the same expert should assess a particular category for all suppliers included. The final assessment report contains an overall rating for each category. Certain criteria may be defined as minimum requirements. They should receive additional comment from the experts as to whether an existing gap could be closed by additional measures. Ideally, the potential suppliers are shown their assessment and the opportunity to provide feedback is given. After the technical assessment, the different results from the rating as well as the written technical assessment report are reviewed again by the external supply integration team, quality and compliance, logistics, and finance, which complements the assessment by the technical experts with a more holistic view. The result is a priority list based on the existing short list of potential suppliers.
Step 5: Bid Execution
The potential suppliers on the priority list are approached again for a quotation based on detailed technical specifications and realistic project goals resulting from the technical assessment. The bid request also includes binding plans and actions required to mitigate gaps which have been identified during the technical assessment, actions necessary to get aligned with general expectations, as well as actions required to achieve the objectives of the collaboration. After bid receipt and comparison, both technical assessment and quote should be compared and a final decision taken. The preferred partner is then invited for a strategy alignment workshop. Partners not selected should be informed accordingly and placed on hold until the selection process is completed, as several hurdles have to be cleared during the strategic alignment as well as the contract signing phase.
Step 6: Strategy Alignment
A strategy alignment workshop is prepared by both parties and should involve middle and higher management representatives. The objective of this workshop is to determine a common strategy for the future collaboration. Vision and mission, targets, communication, relationship management, innovation strategy, supply chain set-up, escalation channels, available resources, etc. are discussed and defined. A relationship charter, relationship governance as well as agreed transition and integration governance should be the outcome.
Step 7: Contracting
The transition process step covers the time period from the signature of the letter of intent, through the actual project phase to ramp up the collaboration, to the transition of the relationship to a functioning level. During this phase a project team composed of members of both parties work on the technical transfer and the finalization of all needed agreements, like the quality, supply and service level and other specific agreements needed to cover and specify the collaboration. After some months, the letter of intent should be replaced with the final contract. This transition phase focuses on process, product and knowledge transfer and ends ideally with an agreed plan for handover to the final integration step. The transition and integration steps have an overlap phase where the transition team maintains responsibility for the final result while the integration team operates the partnership. This overlap phase could be time-bound through agreements made for a certain number of batches, for example. Both transition and integration phase should be managed by a joint leadership team as well as a joint operation management team. Finally, the term sheet and contract are established, incorporating key contractual terms as well as partnership objectives. Important items here are the focus on common goals and deliverables, contract duration and commitments, information exchange and intellectual property, problem solving approach and escalation, open book costing and transparency. The time period from partner selection to the end of the contract is followed by an integration and supplier relationship management process.
4.2 Criteria for partner selection
Basic criteria Basic criteria are the criteria a potential partner has to fulfil as a minimum requirement to qualify for partnerships. Companies formulate their expectations in a statement. An example for basic criteria in the quality and regulatory arena is given by Schönsleben (2007): each partner carries extensive responsibility for end-user satisfaction, and guidelines, structures and processes of the partnership are developed mutually and act as a basis for the first- and second-tier suppliers as well as for the customer relationships and return processes. These basic criteria can be clustered into three categories: 1) quality and compliance criteria, 2) code of conduct criteria and 3) supply chain partnership criteria.
- Quality and compliance criteria: They are quite standardized in this highly regulated industry and often involve widespread industry practices and require full compliance with quality and regulatory requirements, like the International Conference on Harmonization of technical requirements for registration of pharmaceuticals for human use and Good Manufacturing Practice (GMP). The most widespread version of GMP is the one by the World Health Organization which is used by pharmaceutical regulators and the pharmaceutical industry in over one hundred countries worldwide, primarily in the developing world. There are two other popular versions, one by the European Union (EUGMP) and the other by the Food and Drug Administration in the US, referred to as cGMP.
- Code of conduct criteria: They can be subcategorized into labor conditions, health and safety, environment and ethics (Oehmen et al. 2010). Often, full compliance with domestic laws is required, and for labor conditions international standards are applied such as those of the International Labor Organization of the United Nations. Some of the topics that arise here are child labor, discrimination, bribery and conflict of interest. The protection of patent and other intellectual property rights may be of special importance for strategic partnerships in the pharmaceutical industry. An increasing number of companies have prepared a specific code of conduct for their suppliers and strategic partners, usually titled supplier code of conduct (Oehmen et al. 2010).
- Supply chain partnership criteria: A set of qualitative criteria is recommended for the general basic criteria referring to the supply chain partnership. Kim et al. (2010) analyze the critical success factors in supply chain partnerships as discussed in current research. They identify eight factors that fall into the category of enabling criteria: leadership, commitment, coordination, trust, communication, conflict resolution techniques, resources, and performance. Some of them are already covered in the other categories. Other criteria which were of importance were supply chain reliability and business continuity planning, as well as financial liability and stability.
Strategic Criteria
The strategic criteria are company-specific and aligned with the strategy depending on the purpose of the partnership. This study suggests a criteria catalogue using the four categories 1) reach, 2) integration, 3) technology and 4) customer insight. The level of strategic fit will be defined according to these. The criteria mentioned here refer to the partner selection process. For an established strategic supply chain partnership, different criteria have to be applied.
- Reach: Partners with global, regional or local presence and capabilities in the manufacturing and/or distribution of desired products to desired customers in the world, the region or a particular country. Large pharmaceutical companies can benefit from expanding their global reach, reduced cost, supply chain resilience, and secured sales. The main partner benefit is economy of scale.
- Integration: Partners with horizontal R&D and production capabilities enabling rapid new product introduction with the capability to perform clinical trials, registration, submission and commercialization of products. Partners with vertical integration and excellent capabilities in a specific supply chain step like manufacturing of active ingredients, compounding, filling, optical inspection or secondary packaging. The main benefits for the pharmaceutical company are in the case of horizontal integration faster time-to-market and increased sales, and in the case of vertical integration reduced cost. Again, the partner benefits from economy of scale effects.
- Technology: Partners specialized in readily available manufacturing technologies supporting manufacturing platforms, like liquid parenteral, solids tableting, transdermal patches or packaging. Partners with specific manufacturing process capabilities like auto injectors, dual chamber technology or other technologies requiring specialization and capital intense investments. Partners specialized in the management and operation of those technologies. Large pharmaceutical companies benefit from supply chain resilience, life cycle management, reduced cost, and avoidance of tied-up or fixed capital. Partners benefit from high volumes of produced units for their specialized technologies and economy of scale effects.
- Customer insight: Partner with local or regional presence enabling market entry or growth opportunity in a specific market segment, like the branded generics business in emerging countries. As some countries are unique in terms of regulations and market access, local companies could be of strategic help in understanding regional and local specifics both visible and not so visible (for example, some countries in emerging or developing markets do not accept products produced in specific countries). The pharmaceutical company benefits from market entry and the partner from a business model attracting high volumes for specialized technologies and economy of scale.
5 Conclusion
After describing important aspects of implementation, the impact of the partner selection process as well as the limitations and need for further research are explained.
5.1 Implementation of the selection process
Three aspects have to be taken care of and are basic to project management. First is the involvement of all relevant stakeholders. It is a challenge to include all needed stakeholders right from the start while keeping the project team lean and decisive. Secondly, seamless cross-functional collaboration is important. Teams with different interests and views from sourcing, production, compliance, etc. need to talk the same language and work towards the same goal. A culture of openness to compromise and participative leadership will be a great help. But in the end it is also a matter of training. After several projects have been finished, they should be reviewed and the process and the collaboration continuously improved. The same is true for the third success factor, the clear definition of team responsibilities. As mentioned, the involvement of all stakeholders is required, but duplication of work has to be avoided and decision makers should be experts in their fields and not biased by conflicting interests.
Depending on the process phase, different teams are involved and several tools are used to ensure information access and flow. Besides having the right process in place, the appropriate organizational structure has to be established to properly support these teams realizing the partner selection process. Several of the study participants have confirmed that they are increasing their resources for the selection and management of external partnerships within the supply chain department. Furthermore, the result of restructuring approaches of large pharmaceutical companies has set the supply chain department on the same level as internal manufacturing.
An important basis for successful implementation of the process is to make sure that the appropriate market intelligence tools are in place. Access to information and efficient management is important, especially due to the number of different teams involved in the process steps. Market information is extremely important once the RFI process starts, as by then a pre-selection of partners is made. Looking at the linkages of each single process step with the market intelligence function, it is obvious that without a clearly described process and supporting tools a lot of intangible information will be lost and not be visible to the people involved in the initial scouting phase or to people who need information for any other reason or for some future project. Strategic partnerships are also an important means to control risks. Depending on the choice of partners and the type of partnership established, risks can be avoided, shared or transferred. It is also crucial for the implementation to include risk considerations in the overall decision making process and especially the partner selection process.
5.2 Impact of the partner selection process
The study confirmed that strategic outsourcing requires different partner selection processes and selection criteria compared to opportunistic outsourcing as outsourcing has to fit into the whole corporate strategy taking into account all advantages and disadvantages on a corporate level (e.g. risks for the core business). Partnering is shifting away from being purely a matter of cost reduction towards a more strategic partnership approach where partnering is seen as an opportunity to create value for the company.
The short-term impact within the analyzed pharmaceutical company was a significant change in the thinking of the core people. Selecting and establishing strategic partnerships was seen more as a key competence for achieving long-term strategic advantages rather than only achieving short-term cost saving potentials. One concrete aspect was the insight that a long-term relationship enables the parties within a strategic partnership to take more strategic decisions allowing long-term joint investments.
5.3 Limitations and need for further research
The strategic partner selection process for outsourcing of pharmaceutical manufacturing presented in this paper is still rather generic and specific to the pharmaceutical company analyzed and described in the case study. An important question is whether the partner selection process from this specific case can be generalized in the sense that the partner selection process can be used and implemented as best practice process also in other pharmaceutical companies. The possibility of generalization is expected and future research involving other large pharmaceutical companies could confirm that this process can be also adapted to other companies.
The selection process, including the criteria for partner selection, is currently implemented in the analyzed pharmaceutical company, but will need to be adapted to unforeseen aspects. As this process is newly developed, long-term results of its application cannot yet be provided and are a matter for future research. Another topic of future research would be in-depth analysis of the criteria applied in the process. If the research gap as regards measuring financial impact on outsourcing results could be closed, these results would complement and validate the partner selection process.
References
Abdullah, L.M., and Verner, J.M. (2012): Analysis and application of an outsourcing risk framework, Journal of Systems and Software, 85 (8), 1930- 1952.
Akbarzadeh, N., and Pasek, Z.J. (2008): An analytical model for manufacturing service supply contracts, Journal of Manufacturing Systems, 27 (2), 70-76.
Arranz, N., and de Arroyabe, J.C.F. (2008): The choice of partners in R&D cooperation: An empirical analysis of Spanish firms, Technovation, 28 (1- 2), 88-100.
Baines, T.S., Lightfoot, H.W., Benedettini, O., and Kay, J.M. (2009a): The servitization of manufacturing: A review of literature and reflection on future challenges, Journal of Manufacturing Technology Management, 20 (5), 547-567.
Baines, T.S., Lightfoot, H.W., and Kay, J.M. (2009b): Servitized manufacture: Practical challenges of delivering integrated products and services. Proceedings of the Institution of Mechanical Engineers, Journal of Engineering Manufacture, 223 (9), 1207-1215.
Bath, J. (2003): Optimising the value of outsource partners, Contract Services Europe, 12-16.
Bhadoria, V., and Rajpal, J. (2011): Managing pharma supply networks in emerging markets, Pharmacuetical Manufacturing Magazine, available at http://www.pharmamanufacturing.com/articles/ 2011/142.html, accessed April 24, 2013.
Bhakoo, V., Singh, P., and Sohal, A. (2012): Collaborative management of inventory in Australian hospital supply chains: Practices and issues. Supply Chain Management: An International Journal, 17 (2), 217-230.
Bianchi, M., Cavaliere, A., Chiaroni, D., Frattini, F. and Chiesa, V. (2011a): Organisational modes for open innovation in the bio-pharmaceutical industry: An exploratory analysis, Technovation, 31 (1), 22-33.
Bianchi, M., Chiaroni, D., Chiesa, V., and Frattini, F. (2011b):. Organizing for external technology commercialization: Evidence from a multiple case study in the pharmaceutical industry, R&D Management, 41 (2), 120-137.
Booth, R. (1999): The global supply chain. FT healthcare management report, London: Financial Times Business.
Boucher, X., and Afsarmanesh, H. (2013): Modelling collaborative and interoperable enterprise processes, International Journal of Computer Integrated Manufacturing, 26 (11), 988-989.
Braun, I., Pull, K., Alewell, D., Störmer, S., and Thommes, K. (2011): HR outsourcing and service quality: Theoretical framework and empirical evidence, Personnel Review, 40 (3), 364-382.
Burns, R.B. (2009):: Introduction to Research Methods, London: Sage.
Byrne, J. (1993): The virtual corporation. Business Week, 26 (3), 36-41.
Cavalla, D. (2003): The extended pharmaceutical enterprise, Drug Discovery Today, 8 (6), 267-274.
Chen, L.H., and Hung, C.C. (2010): An integrated fuzzy approach for the selection of outsourcing manufacturing partners in pharmaceutical R&D, International Journal of Production Research, 48 (24), 7483-7506.
Chew, I.M.L., Tan, R.R., Foo, D.C.Y., and Chiu, A.S.F. (2009): Game theory approach to the analysis of inter-plant water integration in an eco-industrial park, Journal of Cleaner Production, 17 (18), 1611-1619.
Citron, P. (2012): Ethics considerations for medical device R&D, Progress in Cardiovascular Diseases, 55 (3), 307-315.
Clinkscales, K., and Geimer, H. (2001): Must for managing contract manufacturers, Pharmaceutical Technology Europe, 13, 24-28.
Collins, R., and Bechler, K. (1999): Outsourcing in the chemical and automotive industries: Choice or competitive imperative?, Journal of Supply Chain Management, 35 (3), 4-11.
Contractor, F.J., Woodley, J.A., and Piepenbrink, A. (2011): How tight an embrace? Choosing the optimal degree of partner interaction in alliances based on risk, technology characteristics, and agreement provisions, Global Strategy Journal, 1 (1-2), 67-85.
Côté, R., and Hall, J. (1995): Industrial parks as ecosystems, Journal of Cleaner Production, 3 (1-2), 41- 46.
Coughlan, P., and Coghlan, D. (2002): Action research for operations management, International Journal of Operations and Production Management, 22 (2), 220-240.
Crispim, J.A., and Pinho De Sousa, J. (2009): Partner selection in virtual enterprises: a multi-criteria decision support approach, International Journal of Production Research, 47 (17), 4791-4812.
Diestre, L., and Rajagopalan, N. (2012): Are all ‘sharks’ dangerous? New biotechnology ventures and partner selection in R&D alliances, Strategic Management Journal, 33 (10), 1115-1134.
Ding, R., Dekker, H.C., and Groot, T. (2013): Risk, partner selection and contractual control in interfirm relationships, Management Accounting Research, 24 (2), 140-155.
Ducq, Y., Chen, D., and Alix, T. (2012): Principles of servitization and definition of an architecture for model driven service system engineering, In: Sinderen, M., Johnson, P., Xu, X., and Doumeingts, G. (Eds). Enterprise Interoperability, 117-128. Heidelberg: Springer.
Eden, C., and Huxham, C. (1996): Action research for management research, British Journal of Management, 7, 75-86.
Eisenhardt, K.M. (1989):. Building theories from case study research, Academy of Management Review, 14 (4), 532-550.
Eisenhardt, K.M., and Graebner, M.E. (2007): Theory building from cases: opportunities and challenges, Academy of Management Journal, 50 (1), 25-32.
Ernst & Young (2010): Progression Pharma 3.0 – Global Pharmacuetical Industry Report.
Festel, G., Schicker, A., and Boutellier, R. (2010): Performance improvement in pharmaceutical R&D through new outsourcing models, Journal of Business Chemistry, 7 (2), 89-96.
Festel, G. (2011): Outsourcing chemical synthesis in the drug discovery process, Drug Discovery Today, 16 (5-6), 237-243.
Fields, G. (2004): Territories of Profit: Communications, Capitalist Development and the InnovativeEnterprises of G. F. Swift and Dell Computer. Stanford, CA.: Stanford University Press.
Fujiwara, T. (2013): Real options analysis on strategic partnership dealing of biotech start-ups, Global Journal of Flexible Systems Management, 14 (1), 17-31.
Geng, Y., Haight, M., and Zhu, Q. (2007): Empirical analysis of eco-industrial development in china, Sustainable Development, 15 (2), 121-133.
Getz, K. (1997): The expanding outside clinical services contractor marketplace, Clinical Research and Regulatory Affairs, 14 (3-4), 191–204.
Gilley, K.M., Greer, C.R., and Rasheed, A.A. (2004): Human resource outsourcing and organizational performance in manufacturing firms, Journal of Business Research, 57 (3), 232-240.
Greenwood, D.J., and Levin, M. (1998): Introduction to action research. Thousand Oaks: Sage.
Gu, F., and Li, J.Q. (2010): The value-relevance of advertising: Evidence from pharmaceutical industry, Journal of Accounting, Auditing & Finance, 25 (1), 85-120.
Han, C., Porterfield, T., and Li, X. (2012): Impact of industry competition on contract manufacturing: An empirical study of U.S.manufacturers, International Journal of Production Economics, 138 (1), 159-169.
Henderson, R., and Cockburn, I. (1994): Measuring competence? Exploring firm effects in pharmaceutical research, Strategic Management Journal, 15 (S1), 63-84.
Henderson, R., and Cockburn, I. (1996): Scale, scope, and spillovers: The determinants of research productivity in drug discovery., RAND Journal of Economics, 27 (1), 32-59.
Hess, A.M., and Rothaermel, F.T. (2011): When are assets complementary? Star scientists, strategic alliances, and innovation in the pharmaceutical industry, Strategic Management Journal, 32 (8), 895-909.
Hsiao, H.I., Kemp, R.G.M., Van Der Vorst, J.G.a.J., and Omta, S.W.F. (2010a): A classification of logistic outsourcing levels and their impact on service performance: Evidence from the food processing industry, International Journal of Production Economics, 124 (1), 75-86.
Hsiao, H.I., van der Vorst, J.G.A.J, Kemp, R.G.M., and Omta, S.W.F. (2010b): Developing a decision-making framework for levels of logistics outsourcing in food supply chain networks, International Journal of Physical Distribution & Logistics Management, 40 (5), 395-414.
Isenmann, R., Bey, C., and Keitsch, M. (2008): Beyond a sack of resources. Nature as a model – core feature of industrial ecology. In: Ruth, M., and Davidsdottir, B. (Eds), Changing stocks, flows and behaviors in industrial ecosystems, 157-181. Cheltenham, Northampton: Edward Elgar.
Isenmann, R., and Hauff, M. von (Eds) (2007): Industrial Ecology, Mit Ökologie nachhaltig wirtschaften. München: Elsevier.
Jiang, B., and Qureshi, A. (2006): Research on outsourcing results: current literature and future opportunities, Management Decision, 44 (1), 44- 55.
Jiang, B., Frazier, G.V., and Prater, E.L. (2006): Outsourcing effects on firms’ operational performance: An empirical study, International Journal of Operations & Production Management, 26 (12), 1280-1300.
Jung, J.J. (2008): Taxonomy alignment for interoperability between heterogeneous virtual organizations, Expert Systems with Applications, 34 (4), 2721-2731.
Kakabadse, A., and Kakabadse, N. (2005): Outsourcing: Current and future trends, Thunderbird International Business Review, 47 (2), 183-204.
Kim, B. (2003): Dynamic outsourcing to contract manufacturers with different capabilities of reducing the supply cost, International Journal Production Economics, 86 (1), 63-80.
Kim D.Y., Kumar V., and Kumar U. (2010): Performance assessment framework for supply chain partnership, Supply Chain Management: An International Journal, 15 (3), 187-195.
Kitcher, B., Mccarthy, I.P., Turner, S., and Ridgway, K. (2012): Understanding the effects of outsourcing: Unpacking the total factor productivity variable, Production Planning & Control, 24 (4-5), 308-317.
Kumar, S., Dieveney, E., and Dieveney, A. (2009): Reverse logistic process control measures for the pharmaceutical industry supply chain, International Journal of Productivity and Performance Management, 58 (2), 188-204.
Kumari, S., Singh, A., Mishra, N., and Garza-Reyes, J. (2013): A multi-agent self-adaptive architecture for outsourcing manufacturing supply chain, In: Azevedo, A. (Ed). Advances in sustainable and competitive manufacturing systems, 23rd International Conference on Flexible Automation & Intelligent Manufacturing, 1185-1196. Heidelberg: Springer.
Lai, E.C., Riezman, R., and Wang, P. (2009): Outsourcing of innovation, Economic Theory, 38 (3), 485- 515.
Lau, H.C.W., and Wong, E.T.T. (2001): Partner selection and information infrastructure of a virtual enterprise network, International Journal of Computer Integrated Manufacturing, 14 (2), 186- 193.
Lay, G., Copani, G., Jäger, A., and Biege, S. (2010): The relevance of service in European manufacturing industries, Journal of Service Management, 21 (5), 715-726.
Lee, C.W. (2007):. Strategic alliances influence on small and medium firm performance, Journal of Business Research, 60 (7), 731-741.
Leng, J., Jiang, P., and Ding, K. (2014): Implementing of a three-phase integrated decision support model for parts machining outsourcing, International Journal of Production Research, 1-23.
Li, D., Eden, L., Hitt, M.A., and Ireland, R.D. (2008): Friends, acquaintances, or strangers? Partner selection in R&D alliances, Academy of Management Journal, 51 (2), 315-334.
Li, J., and Hao, Z. (2011): The organization of regional industry ecosystem: Structure and evolution, Industrial Engineering and Engineering Management (IE&EM), IEEE 18Th International Conference on 3-5 Sept. 2011, 773-777.
Linna, A., Korhonen, M., Mannermaa, J.P., Airaksinen, M., and Juppo, A.M. (2008): Developing a tool for the preparation of gmp audit of pharmaceutical contract manufacturer, European Journal of Pharmaceutics and Biopharmaceutics, 69 (2), 786-792.
Liston, P., Byrne, J., Byrne, P.J., and Heavey, C. (2007): Contract costing in outsourcing enterprises: Exploring the benefits of discrete-event simulation, International Journal of Production Economics, 110 (1–2), 97-114.
Lowman, M., Trott, P., Hoecht, A., and Sellam, Z. (2012): Innovation risks of outsourcing in pharmaceutical new product development, Technovation, 32 (2), 99-109.
Macher, J.T., and Boerner, C. (2012): Technological development at the boundaries of the firm: A knowledge-based examination in drug development, Strategic Management Journal, 33 (9), 1016-1036.
MacKerron, G., Kumar, M., Benedikt, A., and Kumar, V. (2014): Performance management of suppliers in outsourcing project: Case analysis from the financial services industry, Production Planning & Control,.
Maes, T., van Eetvelde, G., De Ras, E., Block, C., Pisman, A., Verhofstede, B., Vandendriessche, F., and Vandevelde, L. (2011): Energy management on industrial parks in Flanders, Renewable and Sustainable Energy Reviews, 15 (4), 1988-2005.
Martinez, V., Bastl, M., Kingston, J., and Evans, S. (2010): Challenges in transforming manufacturing organisations into product-service providers, Journal of Manufacturing Technology Management, 21 (4), 449-469.
Mayer, K.J., and Salomon, R.M. (2006): Capabilities, contractual hazards, and governance: Integrating resource-based and transaction cost perspectives, Academy of Management Journal, 49 (5), 942-959.
Moore, J.F. (1997): The death of competition: Leadership and strategy in the age of business ecosystems, New York: Harper Business.
Müller, R., Glückler, J., Aubry, M., and Shao, J. (2013): Project management knowledge flows in networks of project managers and project management offices: A case study in the pharmaceutical industry, Project Management Journal, 44 (2), 4-19.
Naerhi, M., and Nordstroem, K. (2005): Manufacturing, regulatory and commercial challenges of biopharmaceuticals production: a Finnish perspective, European Journal of Pharmaceutics and Biopharmaceutics, 59 (3), 397-405.
Neely, A. (2008): Exploring the financial consequences of the servitization of manufacturing, Operations Management Research, 1 (2), 103- 118.
Oehmen, J., De Nardo, M., Schoensleben, P., and Boutellier, R. (2010): Supplier code of conduct – state-of-the-art and customisation in the electronics industry, Production Planning & Control, 21 (7), 664-679.
Piachaud, B.S. (2002): Outsourcing in the pharmaceutical manufacturing process: an examination of the CRO experience, Technovation, 22 (2), 81-90.
Pero, M., and Rossi, T. (2013): RFID technology for increasing visibility in ETO supply chains: a case study, Production Planning & Control.
Plambeck, E.L., and Taylor, T.A. (2005): Sell the plant? The impact of contract manufacturing on innovation, capacity and profitability, Management Science, 51 (1), 133-150.
PriceWaterhouseCoopers (2011): Pharma 2020: Supplying the future, New York, available at http://www.pwc.com/gx/en/pharma-life-sciences/pharma-2020/pharma-2020-supplyingthe-future.jhtml, accessed April 24, 2013..
Ramani, S.V., El-Aroui, M.A., and Audinet, P. (2001): Technology transfer: Partner selection and contract design with foreign firms in the Indian biotechnology sectors, The Developing Economies, 39 (1), 85-111.
Ren, Y.T., and Yeo, K.T. (2006): Research challenges on complex product systems (cops) innovation, Journal of the Chinese Institute of Industrial Engineers, 23 (6), 519-529.
Ritala, P., Agouridas, V., Assimakopoulos, D., and Gies, O. (2013): Value creation and capture mechanisms in innovation ecosystems: A comparative case study, International Journal of Technology Management, 63 (3), 244-267.
Rodríguez Monroy, C., and Vilana Arto, J.R. (2010): Analysis of global manufacturing virtual networks in the aeronautical industry, International Journal of Production Economics, 126 (2), 314- 323.
Roy, S., and Sivakumar, K. (2012): Global Outsourcing Relationships and Innovation: A Conceptual Framework and Research Propositions, Journal of Product Innovation Management, 29 (4), 513-530.
Sanders, N.R., Locke, A., Moore, C.B., and Autry, C.W. (2007): A multidimensional framework for understanding outsourcing arrangements, Journal of Supply Chain Management, 43 (4), 3-15.
Schönsleben, P. (2007): Integral logistics management operations and supply chain management in comprehensive value-added networks, 3rd edition. Boca Raton: Auerbach Publications.
Schuhmacher, A., Germann, P.G., Trill, H., and Gassmann, O. (2013): Models for open innovation in the pharmaceutical industry, Drug Discovery Today, 18 (23-24), 1133-1137.
Shah, N. (2004): Pharmaceutical supply chains: Key issues and strategies for optimisation,Computers & Chemical Engineering, 28 (6-7), 929-941.
Sharp, B., Atkins, A.S., and Kothari, H. (2011): An ontology based multi-agent system to support HABIO outsourcing framework, Expert Systems with Applications, 38 (6), 6949-6956.
Smith, L., Maull, R., and Ng, I.C.L. (2014): Servitization and operations management: A service dominant logic approach, International Journal of Operations & Production Management, 34 (2), 242-269.
Song, H., and Wang, L. (2009): The status and development of logistics cost management: Evidence from mainland china,Benchmarking: An International Journal, 16 (5), 657-670.
Subramaniam, S., and Dugar, S. (2012): Outsourcing drug discovery to india and china: From surviving to thriving,Drug Discovery Today, 17 (19–20), 1055-1058.
Sun, H., Wan, N., Chang, Z., and Mo, R. (2011): Approach to optimization of part machining service combination, The International Journal of Advanced Manufacturing Technology, 56 (5-8), 767-776.
Susman, G.I., and Evered, R.D. (1978): An assessment of the scientific merits of action research,Administrative Science Quarterly, 23 (4), 582-603.
Urbani, A., Molinari-Tosatti L., and Pasek, Z.J. (2002): Manufacturing Practices in Dynamic Markets: Reconfigurability to Enable a Service-Based Manufacturing Capacity Supply, Proceedings of the ASME International Mechanical Engineering Congress and Exposition. New Orleans: American Society of Mechanical Engineers.
Van Arnum, P. (2006): Outsourcing solid dosage form manufacturing, Pharmaceutical Technology, 30 (6), 44-52.
Vandermerwe, S. and Rada, J. (1988): Servitization of business: Adding value by adding services, European Management Journal, 6 (4), 314-324.
Verhasselt, S., Festel, G., and Schönsleben, P. (2012): Supply-Chain-Strukturen und -Abläufe in der Pharmaindustrie / Aktuelle Trends vor dem Hintergrund einer sich verändernden Umwelt, Pharmind, 2012, 164-170.
Vitasek, K., Ledyard, M., and Manrodt, K.B. (2013): Vested outsourcing: Five rules that will transform outsourcing, 2nd edition, New York: Palgrave Macmillan.
Vitasek, K,. and Manrodt, K. (2012): Vested outsourcing: A flexible framework for collaborative outsourcing, Strategic Outsourcing: An International Journal, 5 (1), 4-14.
Waggener, J. (2003): Conference report: FDA officials discuss current enforcement initiatives: inspections, outsourcing, process analytical technology, consent degrees, technology transfer, aseptic processing, Journal of GxP Compliance, 7, 67-78.
Westbrook, R. (1995): Action research: a new paradigm for research in production and operations management, International Journal of Operations and Production Management, 15 (12), 6-20.
Wilkinson, A., Dainty, A. and Neely, A. (2009): Changing times and changing timescales: the servitization of manufacturing, International Journal of Operations & Production Management, 29 (5), 425-430.
Wittstruck, D., and Teuteberg, F. (2011): Towards a holistic approach for sustainable partner selection in the electrics and electronics industry, In: Nüttgens, M., Gadatsch, A., Kautz, K., Schirmer, I., and Blinn, N. (Eds). Governance and sustainability in information systems, Managing the transfer and diffusion of it, 45-69. Heidelberg: Springer.
Yin, R.K. (2013): Case Study Research: Design and Methods, 5th edition. Thousand Oaks: Sage.
Zhang, M., Pawar, K.S., Shah, J., and Mehta, P. (2013): Evaluating outsourcing partners’ capability: a case study from the pharmaceutical supply chain, Journal of Manufacturing Technology Management, 24 (8), 1080-1101.
Zhen, L. (2012): An analytical study on service-oriented manufacturing strategies, International Journal of Production Economics, 139 (1), 220- 228.
Zolghadri, M., Amrani, A., Zouggar, S., and Girard, P. (2011): Power assessment as a high-level partner selection criterion for new product development projects, International Journal of Computer Integrated Manufacturing, 24 (4), 312-327.