Detergent Phosphates: an EU Policy Assessment
Abstract: Sodium tripolyphosphate (STPP) is an important ingredient of many detergents. The use of STPP has been associated with the environmental problem of “eutrophication”, the increase of nutrient levels in water, which can lead to the formation of large masses of algae or blooms which are unsightly, cause slow moving water to be turbid, and may be toxic. This paper considers policies to reduce the use of STPP in detergents and assesses their success in reducing eutrophication together with the impact on the phosphate industry. The extent of eutrophication has been reduced, but there is still an ecological problem in many areas. Policy directed specifically at detergent phosphates has now been effectively made redundant by the EU requirement to install tertiary treatment plant. While the phosphate industry has experienced a considerable reduction in demand and has consequently contracted, it can be expected to stabilise. Policy on phosphorus will continue to evolve, from the current emphasis on implementing the EU Directive on Urban Waste Water Treatment to dealing with the consequences of this – sludge – and addressing the other main source of phosphorus – agriculture.
1. Introduction
Laundry detergents are an item that appears on everybody’s shopping list; they perform one of the basic household functions. An important ingredient of many detergents is phosphate in the form of sodium tripolyphosphate (STPP). Its introduction in synthetic detergents in 1948 heralded a step increase in performance over the soap-based products that had been used before. Subsequently, the markets for synthetic detergents grew rapidly in Europe and the US and the production of STPP became a significant part of the phosphate industry, although it has always remained a relatively small part of the market in comparison to fertilisers, which account for around 85% of phosphate production.
However, there is an ecological problem. A consequence of the use of STPP in the domestic environment can be an increased level of phosphates in household waste water, which may then contribute to the phosphorus load in rivers, lakes and inshore waters. This can be an environmental issue because of “eutrophication”, the increase of nutrient levels in water, which can lead to the formation of large masses of algae or blooms which are unsightly, cause slow moving water to be turbid, and may be toxic. The emergence of eutrophication as an issue after the introduction of STPP led to the identification of the use of STPP with this environmental problem, which resulted in both changes in consumer perceptions and the development of government and EU policy on phosphates. However, the presence of cyanobacterial blooms and turbidity is still an important environmental issue in many European countries, the USA and Japan [1].
This history provides an interesting case study of the development of environmental policy and its impact on consumers and industry. [2] and [3] discuss the environmental issues arising from the use of phosphates in detergents. There is, however, very little literature on the application of environmental policy to detergents. [4] is an assessment of taxing phosphates in detergents and [5] considers environmental policy on phosphorus, with application to phosphorus recycling. This paper explains why phosphates are used in detergents and considers policy on phosphates in detergents in response to the ecological problem of eutrophication. We concentrate on the EU, using wider international comparisons when they are informative. The success of the policies in addressing eutrophication is assessed, together with the impacts on the detergents market and on the phosphate industry. This enables some conclusions to be drawn about the nature of environmental policy, applicable to other environmental policy issues.
The paper is structured as follows. Section 2 briefly explains why phosphates are used in detergents and looks at alternative chemicals that can be used. Section 3 describes the ecological problem of eutrophication and assesses the role of detergents as a contributor to the phosphorus load. The environmental solutions that are available are introduced. In order to adopt these solutions, government action is required and section 4 looks at the extensive policy response of individual countries and the EU. The success of these policies is assessed. Section 5 considers the impact of the environmental issue of eutrophication and the policy response on the detergent market. Section 6 draws some general conclusions on the nature of environmental policy. It assesses the effectiveness of the policy response and discusses ways in which environmental policy on detergents and phosphorus might effectively address the continuing issue of eutrophication in the future.
2. The use of phosphates in laundry detergents
The reason why STPP is used in detergents is that it performs several very useful functions. No other single chemical product has been found which performs the same combination of functions as a ‘builder’ – a chemical added to soap to improve the performance of the detergent formulation – and contributes so effectively to the performance of modern household detergents, where washing temperatures are low and soiling of the clothes is generally relatively light. STPP performs the following functions [6]:
- As with all complex phosphates, STPP is alkaline, so it counteracts hardness in water. ‘Hardness’ means that the water contains salts such as calcium chloride or magnesium chloride, which will leave crusty deposits on the clothes. Dirt and the textiles may also contain calcium and magnesium ions. STPP reacts with these salts to combine them into other phosphate containing compounds which do not precipitate, so avoiding further deposits of precipitated crystals on the clothes. This has the additional advantage of preventing deposition on the heating elements in the washing machine [7].
- A combination of 50% detergent and 50% STPP provides a more effective washing performance than using 100% detergent, other factors being equal. Condensed phosphates increase the surface activity of the active washing compounds [8]. An additional effect is that the alkaline STPP raises the pH value in the wash liquid (i.e. acts as a chemical buffer), which means that the ions in the dirt and textile fibres become more strongly charged. This in turn leads to increased repulsion between the ions in the dirt and in the textile, thus increasing washing performance. Of all the phosphate compounds, STPP has the greatest synergy in these respects.
- Complex phosphates such as STPP ‘deflocculate’, which means that they break up large particles of e.g. mud or clay into smaller ones. Furthermore, they keep fine particles in suspension in the washing water and prevent them recombining, thus avoiding redeposition on the clothes. Related to this property of deflocculation, they emulsify oily materials, that is they also break up oily masses into smaller particles.
Because of the alkalinity of STPP, it will redissolve Calcium and Magnesium compounds that are present from detergent in previous washes and will reactivate any remaining soap. Therefore, the performance of the detergent is enhanced in this case.
This combination of functions means that phosphates and STPP in particular can play a very important role in the washing process. If phosphates are not used, they must be replaced with some material or combination of materials that performs a similar combination of functions, if the performance of the detergent is to be maintained. In recent years, there has been innovation in new products such as ‘compact’ powders and tablets. These help to prevent the excessive use of detergents. STPP is particularly suitable for use in both of these new types of product.
2.1 Alternatives to Phosphates in Detergents
As will be shown in sections 3 and 4 below, concern about the environmental impact of phosphates in synthetic detergents resulted in the introduction of various controls and restrictions on the use of phosphates in household detergents. This led to a search for alternative builders. Several replacements have been tried:
Sodium citrate
Sodium citrate was utilised as a builder, but it has some disadvantages [9]. It is considerably more expensive than STPP (twice as much at that time) and does not perform as well in removing calcium and magnesium ions. This lower performance is least marked at very low temperatures.
Ethylene diamine tetraacetic acid (EDTA) and nitrilotriacetic acid (NTA)
Both of these chemicals are effective at abstracting calcium and magnesium ions and NTA in particular can largely replace STPP as a builder [10]. However, it does not buffer as strongly as STPP and is less effective as a particle disperser. The main problems with NTA are that there has been some evidence that it is carcinogenic and its great strength in combining with metal ions has caused fears that heavy metals in sewage sludge may be taken up and hence mobilised [10]. This could then result in peak concentrations of heavy metals in rivers and lakes being above regulated level. [11] argue that this latter risk is not significant, but these environmental concerns have resulted in both EDTA and NTA being excluded from EU Ecolabelable automatic dishwasher and domestic laundry detergents.
Zeolite A – polycarboxylate
The most successful alternative has been Zeolite A, a relatively inert substance derived from aluminium oxide [12]. It has a reasonable performance in abstracting calcium and magnesium ions but is limited as a builder. It does not buffer during the washing process and does not prevent redeposition of soil particles in the wash liquid, so it has to be used with a cobuilder, usually polycoarboxylic acids (PCAs). These are oil-based compounds that soften water and keep soil particles in suspension. Zeolite- PCA builders are now used in almost all countries where STPP is no longer used, in particular the USA, Germany and Italy. It is also extensively used in liquid detergents. Its real advantage is that it never been perceived as presenting a serious environmental problem, while providing reasonable performance for modern household detergent powders although concern has been expressed over the impact of PCAs on heavy metals in water sources [13]. It also results in increased volumes of sludge from sewage treatment plants in comparison to STPP. Zeolites and PCAs contribute significantly to volumes of sludge produced by sewage works, probably generating significantly more sludge than detergent phosphates in cases where either sewage phosphorus removal is not necessary, where phosphorus removal is carried out essentially by biological processes or if phosphorus recycling is installed. The inclusion of zeolites in detergents is estimated to increase sewage works sludge production by 15% [14].
Comprehensive life-cycle comparisons of STPP and Zeolite A – PCA have been undertaken for European conditions [12,15]. These found that the overall environmental impact of the two builder systems was roughly equal in both the UK, which has relatively simple waste water treatment and in Scandinavian countries which have very advanced waste water systems. [15] concludes that using STPP exclusively as a builder is the option with the lowest environmental impact in terms of waste water treatment only. This is mainly because Zeolite builders result in a greater volume of sludge from sewage treatment works and because zeolites and PCAs have no recycling value, whereas phosphorus can be usefully recovered and recycled. Although Life Cycle Analysis methodology has evolved since these studies, more recent work [16] has confirmed the coherence of theses studies’ data and conclusions.
In summary, STPP considerably improves performance of detergents. STPP is particularly useful for heavy soiled washes, and is extensively used in industrial laundry detergents as well as in dishwasher detergents, even in countries where it is no longer present in household detergents. There has subsequently been extensive research into alternatives to phosphates and STPP in particular as a builder. There are alternatives of which Zeolite A is the most successful, although it increases sludge volumes in waste water treatment.
3. Eutrophication and Detergent Phosphates
3.1 Eutrophication
The environmental issue associated with phosphates is eutrophication and the subsequent growth of blooms of cyanobacteria and microscopic algae. Eutrophication describes a situation in which a body of water receives an increased supply of plant nutrients which provide the conditions for the rapid growth of these blooms. Both phosphorus and nitrogen are essential chemicals for plant growth, but are only required in very small quantities under ‘natural’ conditions. Therefore, if the supply of either of these nutrients is suddenly increased, the conditions for plant growth will change and the ecosystem will adapt. In particular, given suitable environmental conditions, blooms will form. [17] and [18] provide empirical evidence that phosphorus can be the limiting nutrient determining the extent of blooms. The growth of algae and cyanobacteria also depend on the water temperature and the availability of sunlight for photosynthesis. Warm water temperatures and plenty of sunlight may combine with slow flowing or stationary water to give the conditions under which blooms can grow.
It is important to note that the size of blooms is governed by these different limiting factors. If the water is warm, there is plenty of sunlight and the requisite minerals, but initially a low level of phosphate, then the introduction of phosphate will cause a growth of the blooms. However, if there is already plenty of phosphate and the growth is limited by, say, nitrogen, then the addition of phosphate will not cause any growth. The relationship between biomass and phosphorus has been statistically estimated in the ‘Vollenweider model’, but because of the other potentially limiting factors, there is no continuous function between biomass and the quantity of phosphate [19]. [20] report that while blooms are associated with eutrophication, there is generally a low correlation between cyanobacterial biomass and total phosphorus or total nitrogen. [21] consider that biological productivity cannot be accurately predicted by simple phosphorus load approaches.
Algae and cyanobacterial blooms are a problem for the environment. The growth of large masses of these blooms may lead to the deoxygenation of deeper waters, threatening rare fish species and invertebrates. Reeds and other submerged plants may be lost and there can be indirect effects on herbivorous bird species. There is thus a loss of the variety of habitat and hence diversity of species [22]. The blooms may also block water filtration systems. Cyanobacterial blooms in particular may have an offensive odour and colour, forming noxious scums and may be toxic [23]. [24] report an incident at Rutland Water, UK in 1989 in which a total of 15 dogs and 20 sheep died after drinking contaminated water. [25] report an incident at Rudyard lake, Staffs., UK where a group of soldiers suffered from gastro-intestinal ailments, one from hallucinations and another from atypical pneumonia. In 1989, 169 water bodies in England and Wales were considered to have problems with cyanobacteria and 68% of 78 sites tested were found to have cyanobacterial toxins [26].
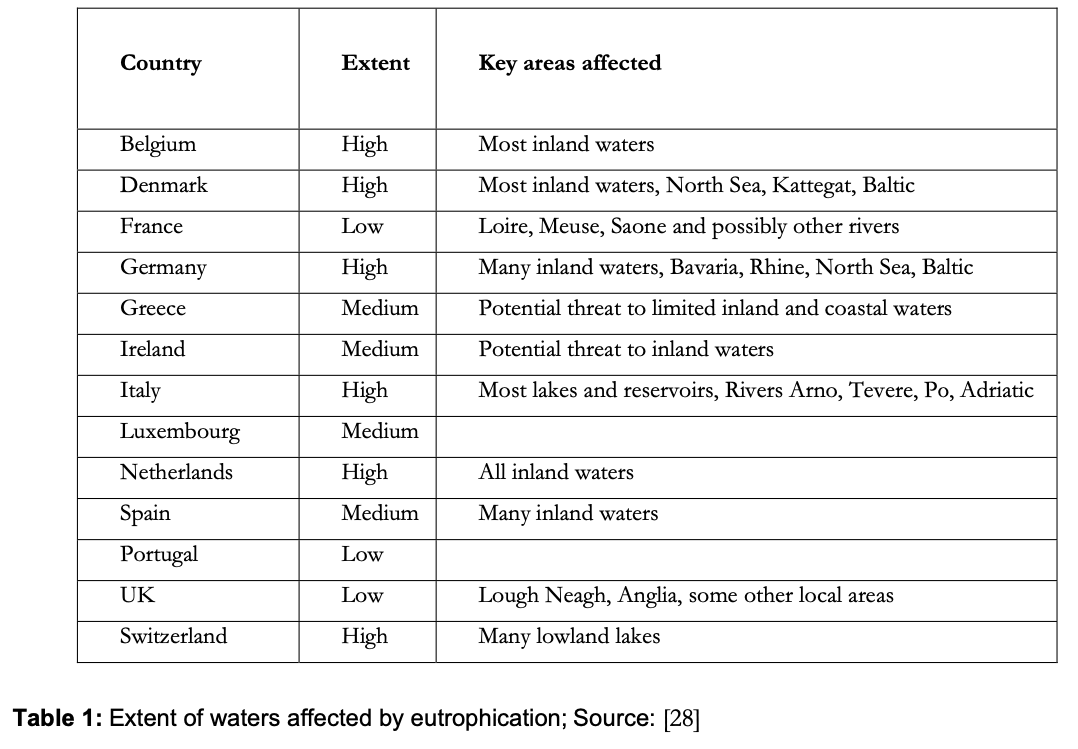
However, [22] consider that in the UK, the growth of cyanobacterial blooms is localised, rather than a widespread problem. They also report that the incidence of environmental problems due to eutrophication in the UK was at a similar level in the 1980s compared with the 1970s. Up to 1989, 16 European countries had reported blooms [27] and blooms have been reported in Australia, Canada, Japan, South Africa and the USA [23]. [28] summarised the situation in Europe at the beginning of the 1990s in table 1:
A recent international survey [1] surveys the wider international context and concludes that phosphorus pollution remains a significant issue in both developing countries and the EU, US and Japan.
3.2 The contribution of detergent phosphates to phosphate loading
Detergent phosphates are a significant, but secondary, source of phosphates in rivers and lakes. Humans and animals are by far the most important sources. [28] state that of phosphorus input to the aquatic environment in the EU, the most important contributors are livestock waste (34%), human waste (24%) and agricultural fertilisers (16%). Detergent phosphates form 10%. [29] state that for waste water input to sewage plants, the most important source is human waste, detergents form between 9% and 50% and that manufacturers’ estimates were 40%; a current manufacturers’ estimate is 20-25%; and industrial processes contribute 9% in the UK. [30] states that detergents led to 40% of water ‘over-fertilisation’ in Austria. Between 30% and 90% of phosphorus loading in rivers is from non-point sources i.e. agriculture[31] and this range is confirmed by [32] who state that in the Minnesota River, US, non- point sources contribute 35% of phosphate loading with low rainfall up to 90% loading in high rainfall. There are some surface waters in which the phosphorus load is dominated by point sources. In 1989 the river Po, Italy, received 67% of phosphorus from point sources and 29% from agriculture; the German Rhine received 77% from point sources and 23% from agriculture in 1985. For 1989-1992 in all of (West) Germany, 52% came through point sources and 42% from agriculture [33].
The conclusion to be drawn from this evidence is that detergent phosphates may indeed lead to eutrophication and the consequent health hazards and degradation of ecosystems. However, the conditions under which these problems arise are limited and inherently site specific. The contribution of household detergents to the total phosphate load that finds its way into rivers, lakes and reservoirs varies considerably. Where phosphorus loading is dominated by waste water inputs, phosphorus from detergents might contribute up to 25% or so of the phosphorus loading. It is not possible to determine in general whether the removal of a certain amount of phosphate will reduce the incidence of blooms or whether an increase in phosphorus loading will cause blooms to develop or grow.
3.3 Solutions to eutrophication involving phosphorus – and their difficulties
Where eutrophication has been caused by phosphorus loading, it can be removed by reducing the phosphorus load, although ecosystems can show considerable hysteresis in their response if the algal blooms have killed off the original species. Although the largest source is agriculture, since phosphate removal from diffuse sources is impracticable, the approach to controlling these sources has to be one of managing the initial use of fertilisers and the careful use and/or disposal of manure [34]. Given this difficulty, there were two relatively obvious courses of action: the restriction of phosphates in household detergents and the treatment of waste water in sewage plants to remove phosphorus. The adoption of alternatives to the use of STPP in detergents has happened to a considerable extent in the EU, as is discussed below. The other obvious possibility is to treat waste water at sewage plants to reduce the phosphorus content of discharges. Treatment of urban waste water to remove phosphorus has a greater potential to change the output into rivers and lakes, because it works on all the phosphate content of waste water instead of only the small fraction from detergents. Also, phosphate removal in sewage works is generally installed at the same time as nitrogen removal, thus also reducing the input of this nutrient to surface waters. Phosphorus technologies are extensively covered in [35]. The technology exists to remove 95% or more of phosphorus from waste water, if the most comprehensive (tertiary) treatment systems are fitted [36].
However, phosphorus removal using the current commercial processes results in the production of phosphorus rich sludge, which must then be disposed of. [35] reviews the state of the art in recycling technologies. Recycling by spreading sludge as fertiliser is the most effective environmental option in many cases [37]. However, in some regions where there is a high population density and limited agricultural land, there may be more sludge produced with high levels of removal than is required for agriculture. Sludge is bulky and therefore has high transport costs, so there must be a demand close to the sewage treatment plant if the economic cost is not to be high. While this is in general the case in the UK, it is not so in many parts of Europe and the US [38]. Most sludge will be produced in urban areas and would have to be distributed to the agricultural areas. This has been found to be the case in the Netherlands and in a study in the Lothian region of Scotland [39]. There are also serious concerns about the concentrations of heavy metals in sludge from waste water, which would also be of concern if their use in fertilisers increased the metal concentration in the food chain, to the extent that the European commission has proposed much stricter controls on the composition of sludge used for spreading [40].
An alternative is to extract the phosphorus from the waste water stream in such a form that it can be reused, either in agriculture as a fertiliser or by phosphate manufacturers as a substitute for the raw material, phosphate rock. A further possibility is to dry and incinerate the sludge, but this requires careful control over the combustion products containing heavy metals, mercury, dioxins and furans, acid gases, as well as NOx and N2O [41]. Another possible use is to dry the sludge and use it for construction materials. In Tokyo, it is used for pavement construction [4].
4. Policies on detergent phosphates, their success and impacts on the phosphate industry
The potential factors causing eutrophication – i.e. increased input of nitrogen and phosphates into watercourses, lakes and seas – were rapidly identified. The increase in phosphorus input due to the introduction of synthetic detergents was perceived to be a major contributor to eutrophication and led to the development of policy to control phosphorus discharges. There is a large literature on environmental policy; initially governments concentrated on limiting pollution by imposing regulations or standards on firms, but the emphasis has moved towards policies employing economic incentives, in particular environmental taxation. [42] surveys the EU experience. Policies on phosphorus have followed this pattern, starting by setting national standards through regulation, through international agreements where necessary. Most policy on phosphates has been of this nature. Germany and the Netherlands are among the signatories to the Rhine Action programme, which required a 50% reduction in inputs of phosphorus and nitrogen to surface waters [43]. With the more recent trend towards the use of economic instruments, such as taxes and charges, the taxation of phosphates is now being considered and has been enacted for detergents in France in particular. There have also been many voluntary agreements to reduce phosphate use in detergents and these have led to significant reductions in phosphate use.
4.1 Command and Control instruments and voluntary agreements between governments and industry to restrict detergent phosphate
Phosphate levels in detergents and also in fertiliser input to agriculture can be specified by legislation or administrative instruments. Given that the main contributor is human waste, which as a biological function cannot be reduced, controlling phosphates in detergents offered the best way of reducing phosphate input into urban waste water. The response of individual countries has depended on the severity of eutrophication and its geographical extent. In 1985, Italy introduced a restriction of 4% STPP content in household detergents (a low enough proportion to prevent effective use of STPP) in negotiation with industry. This was followed by regulatory bans on phosphates in household detergents in Switzerland and Norway and subsequently Austria in 1994. Many US states introduced bans in the early 1990s and Japan also discontinued the use of STPP in detergents. In some countries such as Germany and Italy, and more recently Ireland, a voluntary agreement to reduce STPP use is in effect equivalent to a “ban” of phosphates in household laundry detergents. In most other European countries, and in some EU Accession countries, voluntary agreements are in place limiting detergent phosphate levels to the minimum necessary for phosphates to play an effective role in the detergent.
However, since cyanobacterial blooms and algae are still a widespread problem in Italian surface and coastal waters, regulating detergents in this way is not always effective. [28] found that there were no examples of phosphate limits in detergents making any large impact on eutrophication and [44] concluded that moving to phosphate free detergents would not measurably change phosphorus inputs from the river Redon into Lake Geneva.
4.2 Ecotaxation of detergent phosphates
The modern trend in environmental policy has been to move towards the use of ‘economic instruments’: taxes/subsidies and tradeable permit systems. These types of measures have long been proposed by economists, because under suitable conditions they offer the possibility of taking the costs of pollution abatement into account as well as the pollution reduction and therefore being economically efficient. The idea is that if there are external costs of e.g. pollution to society imposed by some activity, such as the use of detergents, then the user of the detergent should face costs that reflect the external costs. Taxes are usually most appropriate where there is a tax collection system already in place. The advantage is that the tax provides an economic incentive for polluters to change their behaviour and each individual polluter can choose their level of abatement and hence the amount of tax they pay. Thus it is easy to take account of differences in abatement costs between different polluters. In the context of eutrophication, a national tax has the disadvantage that it does not allow for the difference in requirements for the reduction of phosphates between water basins, so the effects may be insignificant or inappropriate in some areas. The only example of a tax on detergents is the French TGAP, discussed below. Taxation of other polluting activities is much more common, both in Europe and in other OECD countries; [42] surveys European environmental taxes. Belgium and the Netherlands have introduced surplus manure charges, which are based on the emissions of phosphorus and/or nitrogen in excess of the environmentally acceptable manure loads per hectare. Norway, Sweden and the USA have introduced fertiliser charges which are taxes on products rather than taxes directly related to the pollution caused.
The fundamental principle of ecotax design is that it should provide an incentive for the polluter to change their behaviour in a way that reduces the undesirable or polluting activity, in this case eutrophication. [45] considers environmental tax design. Usually, a more effective tax requires more measurement or more complex charging schedules. Another factor is the use of the revenues generated by a tax. The revenues may be designated for use in the same area from which the tax is raised (known as earmarking) e.g. paying for the installation of water treatment or they may be used to reduce other taxes such as employment taxes to improve the overall efficiency of the taxation system. [45] argues that the earmarking of tax revenues does not have an economic justification, but is used to make the introduction of a tax more acceptable.
In the case of detergents, the use of a national tax on detergents containing phosphates is problematic for several reasons. In many areas, the main phosphate loading will come from agricultural sources, so a detergent tax does not address the problem. Even if the main phosphorus load comes from urban waste water, since detergents contribute a small proportion of phosphates, a reduction in detergent use will not prevent eutrophication in most cases. It should also be noted that demand for detergents is relatively inelastic; consumers will always wish to wash clothes and will not be very sensitive to changes in detergent prices. Household expenditure on detergents is also a small proportion of expenditure: [46, 47] shows that expenditure on all cleaning and maintenance products is between 0.5% and 1.5% on average in most EU countries, so even a considerable increase in price of detergents is unlikely to cause consumers to use much less. Therefore, an extremely high rate of tax on phosphate detergents would be necessary to have any significant impact on the incidence of cyanobacterial blooms and algae.
The French TGAP
The one example of a tax specifically on phosphates in household detergents is the French ‘Taxe Générale sur les Activités Polluantes (TGAP)’, which came into force in January 2000. The TGAP contains several different taxes on various activities which are seen as polluting. These include: activities modifying water movement and flow, gravel extraction, industrial outputs of heated water and radioactivity, pesticides (but not fertilisers) and laundry detergents. The stated objective of the TGAP is to reduce polluting activities through an improved application of the polluter pays principle and the raising of revenues to finance the 35 hour week and hence employment [48]. The part of the tax applied to detergents is levied on the sales price to the consumer as follows [49]:
Detergents with:
- less than 5% STPP – 470 FF/tonne
- STPP 5-30% – 520 FF/tonne
more than 30% STPP – 570 FF/tonne
This represents 2.35-2.85 FF for a 1 kilo standard detergent packet, which is approximately between 2-3% of the sale price for concentrated powders and 10% for the cheaper powders. The expected revenue for the first year is 500 million FF, out of a total of 4 billion FF for the TGAP. Applying the criteria for efficient taxes outlined above, it can be seen that the TGAp detergent tax does apply directly to the issue of concern i.e. the presence of detergents in urban waste water. The collection of the tax will be relatively simple, because the systems in place for VAT on consumer products can be used. The variation in rates of 0.5 FF or between 0.4% and 2% per packet is probably so small that any consequent reduction in sales in not detectable. These price variations are certainly much smaller than price differences between different products and promotions etc. However, it will probably not achieve its environmental aims of reducing Cyanobacteria blooms and algae in surface waters. As discussed above, STPP is a small part of the phosphate load and so this marginal additional load will only be significant in a small number of cases. As the change in detergent use due to this tax will be small, the change in STPP input into urban waste water will also be small. Detergent STPP forms 9-50% of the phosphate input to waste water and a maximum 25% of the input to rivers, lakes and reservoirs. Assuming the maximum of the range, 50%, if there is a 5% reduction in detergent use, then there would be a 2.5% reduction in STPP input into waste water and a 1.25% reduction in phosphate input to surface waters as a maximum where phosphate loading was dominated by urban waste water. Even for waters sensitive to the phosphate load, this is very unlikely to have any great effect on the growth of cyanobacterial blooms.
4.3 Waste water treatment
Denmark, Germany, the Netherlands, Switzerland and Sweden have all installed a large number of phosphate removal systems. A far- reaching step was taken by the EU in 1991 with adoption of the Directive on Urban Waste Water Treatment (Directive 91/271/EEC), which required installation of phosphorus removal at waste water treatment plants such as to remove most of the phosphorus [5]. This is resulting in the widespread installation of tertiary treatment plant. The new Water Framework Directive [50] maintains the requirements of existing Directives (e.g.91/271) as the minimum baseline to be developed at the catchment level. Since the growth of cyanobacterial blooms and algae is very dependent on the local conditions, a more integrated approach to nutrient load at a local level offers the possibility of more efficient and effective action.
The implementation of this directive, which came into force in 1991 with the requirements regarding phosphate removal applicable by 31/12/1998, is assessed in [36]. Progress has been variable in the different member states, both in the designation of sensitive areas that require phosphate removal and in the installation of treatment systems. While some processes could produce revenue from selling nutrient rich sludge for use as fertiliser or by recycling to phosphorus to phosphate manufacturers, this is not currently profitable [3]. Therefore, the finance must be raised either from public sources or through water charges. This will explain the delay in implementing the EU directive in some cases. However, the costs have not prevented countries such as Germany and Switzerland from installing many phosphate removal plants, so the costs are probably not a major barrier where eutrophication is perceived to be a serious problem.
4.4 Current incidence of eutrophication
While phosphate discharges have been reduced, phosphate concentrations are far above their natural levels in many areas in Europe and eutrophication continues to be an environmental issue. The most recent available evidence demonstrates that eutrophication is still a problem in many parts of the EU, in spite of a considerable history of policy measures over the last 25 years. [51] found that only 10% of 1000 river measurement sites across Europe had phosphorus concentrations below 50 μg/l (the natural background maximum). It has been found that the cyanobacterial blooms may be extremely stable, especially in shallow waters, so that reduction of phosphorus input alone will not restore the waters [52]. There have been some successes. For example in Lake Veluwe, the Netherlands, the installation of phosphate removal in the sewage works discharging into the lake in 1979 and additional flushing in 1985 enabled the lake to recover by the early 1990s, 10 years after the reduction in nutrient loading [53]. The Swiss policy of waste water treatment has also had some success in reducing the incidence of cyanobacterial blooms and algae, in particular in Lakes Geneva and Neuchatel [54]. [51] shows that mean phosphorus concentrations in European rivers generally decreased between 1987-91 and 1992-96 in Western Europe and in some countries of Eastern Europe. However, there are still many sites with very high phosphate concentrations. [51] also states that reductions in phosphorus loading from sewage works now need to be followed by reductions in loading from agriculture, as this is now relatively more important.
Although full compliance with the EU directive still requires far more extensive phosphate removal, [55] considers that the number of heavily polluted rivers in Western Europe has fallen from 25% in 1975-80 to approximately 5% in 1992-98, especially because of the installation of waste water treatment following the Urban Waste Water Directive. This will have been very effective where there was a high proportion of the phosphorus load from point sources, as in the river Rhine.
If countries such as Italy (where waste water treatment is relatively limited and there are serious problems with cyanobacterial blooms and algae) move towards compliance with the Directive by installing more treatment plants, eutrophication due to phosphorus should decrease and this might have a considerable effect on the overall incidence of eutrophication. Sweden, Switzerland and the Great Lakes region of the USA, for example, have implemented phosphate removal programmes, which have controlled the extent of eutrophication [15]. In Sweden it has therefore not been considered necessary to put any controls on phosphates in detergents. EU policy is now changing towards a water catchment based and hence localised approach, within the context of national and EU policy.
5. Market impacts
The understanding of eutrophication and subsequent policy has had a considerable impact on the market for detergents and detergent phosphates. We consider here evidence on consumer reactions and then look at the consequences for firms in the detergent market, from the viewpoint of the phosphate industry.
5.1 Consumer reactions and trends in phosphate use
Laundry detergents are an essential consumption item that is used very regularly and purchased frequently. The emergence of eutrophication as an environmental issue together with increased consumer awareness of environmental issues increased the perception of phosphates in detergents as environmentally damaging resulted in the policies described above. The consumer choice literature on detergents reflects this situation, in which phosphates are mentioned as an environmental issue [4]. In Denmark, Germany and the Netherlands, the market for detergents containing STPP disappeared, while in countries such as France and the UK, there is also a widespread opinion that phosphates are bad for the environment [4]. Since the detergent market is very competitive and marketing oriented, the consequence of this has been that detergent manufacturers have reduced the use of STPP in detergents. In France, detergents contained 24% STPP on average in 1985 which was reduced to 10% in 1998 [4]. ‘Eco- friendly’ detergent brands were introduced in response to this perception, but this was a temporary phenomenon, as the major manufacturers changed their main products to use less STPP anyway [56]. In Europe, the USA and Japan, the use of STPP in detergents has either been stopped or has fallen very considerably and is continuing to be reduced. In other countries such as Russia, China and Latin America, where there are also potentially large consumer markets, the use of detergents is increasing generally and there is little tendency to try and minimise the use of STPP [56,57].
5.2 Implications for the detergent phosphate industry
Position in the supply chain and detergent market conditions
Phosphates are an intermediate product in the detergent supply chain. The production of STPP is based on phosphorus rock as the raw material from which phosphoric acid is manufactured (although, as mentioned above, the technology also exists to recycle phosphates). STPP is sold to detergent manufacturers and detergents are sold through retail outlets, mainly grocery stores/supermarkets. The consumer detergent market is very concentrated, both in retail and in supply. In 1998, Proctor & Gamble and Unilever, the two largest firms in the market, had over 75% of the UK powder detergent market [57]. The retail market is large; expenditure on fabric cleaning products in the UK was £1.18 billion in 1998 with a further £98 million on machine dishwashing products [57]. It is mature; overall demand is roughly constant, although there has been a slow long term decline in volumes in Europe. This is due to fewer people being employed in manual labour and improved performance of detergents. Required quantities have decreased from 200g detergent/wash to 70- 80g/wash, with lower washing temperatures, shorter wash cycles and a lower water use. Therefore, competition is intense with the manufacturers spending heavily on advertising (£76.8 million for fabric detergents in 1998, [57]) and innovation in new products such as ‘compact’ powders and tablets. The lifetime of a detergent formulation is only of the order of one year [4]. STPP is particularly suitable for use in both of these new types of product, so some increase in the use of STPP as these product types develop can be expected. The market for dishwasher detergents, in which STPP is usually used as the builder, is expanding but was only 22% of the laundry detergent market in 1998 [57].
Structure of the detergent phosphate industry
The STPP industry is also heavily internationally concentrated, with the main manufacturers being part of international industrial chemical companies [48]. There is also overcapacity in the European phosphate industry. After STPP was introduced by Procter and Gamble in 1948, the market and production increased rapidly until most countries had at least one manufacturer. The issue of eutrophication and the subsequent bans and restrictions then caused a rapid decline in the industry, with many plants being closed up to 1992 [4]. This led to consolidation of the industry into five producers in Europe. Two of the largest companies have recently been combined; Rhodia took over Albright and Wilson plc. and now has roughly 50% of the European manufacturing capacity [4]. The concentration has been associated with cutbacks in capacity, the latest of which is that Rhodia UK recently announced the closure of 2 of the 3 UK STPP plants with 300 redundancies. The reduction of 140,000T of effective capacity will mean that plants in Europe will improve from operating at 50-55% capacity to over 80% capacity [58]. [48] estimates the turnover of the sole STPP plant in France at 350Mn FF/year, with 150 employees [4].
Internationally, there are detergent markets which might expand. China is the best example, but there is plenty of recently installed manufacturing capacity. There is a relatively low level of detergent consumption in Russia and Eastern Europe and little use of zeolites as a builder so there is potential for growth there [4]. Latin America and South East Asia are also potential markets, although as STPP and powder detergents are quite difficult to transport, it is more probable that local manufacturing plants will be constructed.
Overall, the issue of eutrophication caused a decline in demand for detergent phosphates, resulting in a contraction of the industry and considerably reduced production volumes. There are no large new potential markets outside the EU, although new products such as compact powders and dishwasher detergents may stabilise demand for detergent phosphates.
6. Has policy on detergents been effective and what can future policy on phosphorus achieve?
As the discussion in section 4 above shows, policy to control phosphorus has reduced the extent of eutrophication, but there is still an ecological problem in many areas. Policy directed specifically at detergent phosphates has now been effectively made redundant by the EU requirement to install tertiary treatment plant, which removes most of the phosphorus in urban waste water, of which only a small proportion now comes from detergents. So, while policies on detergents may have reduced the environmental impact of detergents in some cases of eutrophication in the past, this would only be the case in a few particular situations in the future. The impact on the detergent market overall has been small, but in terms of phosphates in detergents, policy and market pressures have acted to considerably reduce the use of STPP. This has led to overcapacity in STPP production, with resultant consolidation of the phosphate industry. Because the industry is highly concentrated, there were considerable economic impacts where plants have been closed, but these are relatively few in number. New products and a move to waste water treatment as the main policy to control phosphorus loading should mean that the industry stabilises.
This case study of the history of environmental policy demonstrates three points. Firstly, sources of pollution from industry or consumer products are easily identified and are relatively easy for policy to influence. However, what is thought of as the main cause of deterioration in an ecosystem is often only the main cause in some instances. Secondly, because ecosystems have non-linear responses to changes in inputs, removing the last source of pollution that ‘tipped the ecosystem over the edge’ will often not return the ecosystem to its previous state, more drastic action may be necessary. Thirdly, the most effective policy may change over time. In the case of detergents, restrictions on phosphates in detergents have been effectively replaced by a requirement to remove phosphorus from urban waste water streams. This will, in turn, lead to changed policy requirements in the future.
In particular, the sludge produced by waste water treatment will have to be dealt with and phosphorus loading from agriculture will become a more serious issue as the main remaining source of phosphorus. [35] surveys phosphorus recycling and [5] looks at policy for recycling phosphorus. This will be costly and require the development of new markets in sludge collection, transport and spreading or treatment. Since phosphorus in urban waste water is now being reduced, future policy will have to address the role of agriculture in contributing to phosphorus load into rivers, lakes and inshore waters. Because agriculture is a diffuse source of phosphorus compared to waste water outlets, its control is more difficult. Policy can provide incentives by taxing excess fertiliser and manure use, as in Belgium and the Netherlands [42]. A further possibility would be to recycle phosphate from animal manure, which could become economically attractive in areas of intensive livestock production [38]. There is a potential synergy with waste water treatment here: if a market for recycled phosphorus from waste water treatment plants is developed, it would be much easier for farms to locate a demand for their recycled phosphorus. [34] also make the point that since the main cause of high phosphorus loading problem is intensive livestock farming, policies to encourage mixed farming will also reduce the incidence of eutrophication.
To summarise, environmental policy is demonstrating some success in reducing the incidence of eutrophication. Full implementation of current EU policy can be expected to reduce eutrophication further. While the phosphate industry has experienced a considerable reduction in demand due to the recognition of the problem and has consequently contracted, it can be expected to stabilise. Policy on phosphorus will continue to evolve, from the current emphasis on implementing the Directive on Urban Waste Water Treatment to dealing with the consequences of this – sludge – and addressing the other main source of phosphorus – agriculture.
Acknowledgement
This paper is based on work undertaken for the Centre Européen d’Etudes des Polyphosphates – a European Chemical Industry Council (CEFIC) sector group. The author wishes to thank two anonymous reviewers for their comments on a previous draft.
References
[1] Farmer, A. (2004), Phosphate Pollution: a Global Overview of the Problem in: E. Valsami-Jones (ed.). Phosphorus in Environmental technologies: Principles and Applications. London: IWA.
[2] Kolber, E. (1990), Detergents, the Consumer and the Environment. Chemistry & Industry, No.6, 179-181.
[3] Driver, J., Lijmbach, D. and Steen, I. (1999), Why recover phosphorus for recycling, and how?. Environmental Technology, 20(7), 651- 662.
[4] Köhler, J. (2001), Detergent phosphates and detergent ecotaxes : a policy assessment, a report prepared for the Centre Européen d’Etudes des Polyphosphates – a European Chemical Industry Council (CEFIC) sector group.
[5] Köhler, J. (2004), Phosphate recycling: Regulation and Economic Analysis in: E.Valsami-Jones (ed.). Environmental technologies: Applications, London: IWA.
[6] Davidsohn, A. and Milwidsky, B.M. (1978), Synthetic detergents 6th ed. London: Godwin; New York: Wiley.
[7] Merkenich, K. and Gohla, W. (1979), Seifen Öle Fette Wachse, 105, p.39.
[8] Ullman (1999), Ullman’s Encyclopedia of Industrial Chemistry 6th Edition. Wiley- VCH, Weinheim.
[9] Stinson, S.C. (1987), Consumer Preferences spur innovation in Detergents. Chemical and Engineering News, Jan 26, 24-46.
[10] Perry, R., Kirk, P.W.W., Stephenson T. and Lester, J.N. (1984), Environmental Aspects of the use of NTA as a Detergent Builder. Water Research 18(3), 255-276.
[11] Brouwer, N.M. and Terpstra, P.M.J. (1995), Ecological and Toxicological Properties of Nitrilotriacetic Acid (NTA) as a Detergent Builder. Tenside Surfactants Detergents, May-Jun, 225-8.
[12] Landbank (1994), The Phosphate Report. Landbank Environmental Research and Consulting, London.
[13] CES (Consultants in Environmental Sciences Ltd.) (1991), Pollutants in Cleaning agents: Final report). Department of the Environment, March 1991, London.
[14] French Regional Water Agency (FRWA) (1996), Note Technique n°2 – SDAGE Rhône-Métierranée-Corse, 12/1996. French regional water agency, Paris.
[15] Landbank (1995), The Swedish Phosphate Report. Landbank Environmental Research and Consulting, London.
[16] EMPA(1999),Lifecycleinventoriesforthe production of detergent ingredients. EMPA report No. 244, EMPA, St. Gallen.
[17] Jones, S.H. and Alexander, M. (1989), Phosphorus enhancement of mineralisation of low concentrations of 4-nitrophenol by Flavobacteriumn sp. in lake water. FEMS Microbiology Letters, 52, 121-36.
[18] Haas, C.N., Bitter, P. and Schoff, P. (1988), Determination of the limiting nutrients for indigenous bacteria in Chicago intake water. Water, Air and Soil Pollution, 37, 65-72.
[19] Reynolds (1992), Eutrophication and the management of plankton algae: what Vollenweider couldn’t tell us. In: D.W.Sutcliffe and J.G.Jones (eds.) Eutrophication: research and applications to water supply, 4-29.
[20] Canfield, Jr. J.E., Phlip, S.E. and Duarte, C.M. (1989), Factors influencing the abundance of blue-green algae in Florida lakes. Canadian Journal of Fisheries and Aquatic Science, 46 1232-7.
[21] Göde, H. and Gries, T. (1998), Phosphorus fluxes in Lake Constance. Archives of Hydrobiology: special issues advanced limnology, 53, 505-544.
[22] Lund, J. W. G. and Moss, B. (1990), Eutrophication in the United Kingdom: trends in the 1980s. Report for the Soap and Detergent Industry Association, London.
[23] Howard, A. (1994), Problem Cyanobacterial Blooms – Explanation and Simulation Modeling. Transactions of the Institute of British Geographers, Vol.19, No.2, 213-224.
[24] Kelly, D.F. and Pontefract, R. (1990), Hepatorenal toxicity in a dog after immersion in Rutland Water. Veterinary Record, Nov. 3rd 453-4.
[25] Turner, P.C., Grammie, A.J., Hollinrake, K. and Codd, G.A. (1990), Pneumonia associated with contact with cyanobacteria. British Medical Journal, 300 1440-1.
[26] NRA (1990), Toxic Blue-Green Algae. National Rivers Authority, London.
[27] Lawton, L.A. and Codd, G.A. (1991), Cyanobacterial (blue-green algal) toxins and their significance in UK and European waters. Journal of the Institute of Water Engineering and Management, 5, 460-5.
[28] Morse, G. K., Lester, J.N. and Perry, R. (1993), The economic and environmental impact of phosphorus removal from wastewater in the European Community. London: Selper.
[29] UK Water Industry Research (UKWIR) (1997), Influence of phosphate on bacterial growth in water systems. Report no.97/DW/02/11, London.
[30] Kolber, E. (1990), Detergents, the Consumer and the Environment. Chemistry & Industry, No.6, 179-181.
[31] Sharpley, A. N., S. C. Chapra, R. Wedepold, J. T. Sims, T. C. Daniel, and Reddy,. K. A. (1994), Managing agricultural phosphorus for protection of surface waters: Issues and options. Journal of Environmental Quality 23:437-451.
[32] McCann, L. M. J. and Easter, K. W. (1999), “Differences between Farmer and Agency Attitudes Regarding Policies to Reduce Phosphorus Pollution in the Minnesota River Basin.” Review of Agricultural Economics 21(1), 189-207.
[33] European Environment Agency (EEA) (1998), Effects of excessive anthropogenic nutrients in European ecosystems, EEA, Copenhagen.
[34] Parr, W., Andrews, K., Mainstone, C.P. and Clarke, S.J. (1999), Diffuse Pollution: sources of nitrogen and phosphorus. DETR report WS 128/1/115, Foundation for Water Research, Marlow, UK.
[35] Valsami-Jones, E. (ed.) (2005), Phosphorus in Environmental technologies: Principles and Applications London: IWA.
[36] IEEP (1999), Implementation of the 1991 EU Urban Waste Water Treatment Directive and its role in reducing phosphate discharges. Institute for European Environmental Policy, London.
[37] Edge, D. (1999), Perspectives for nutrient removal from sewage and implications for sludge strategy. Environmental Technology, 20(7), 759-763.
[38] Greaves, J., Hobbs, P., Chadwick, D. and Haygarth, P. (1999), Prospects for the recovery of phosphorus from animal manures: a review. Environmental Technology, 20(7), 697-708.
[39] Towers, W. and Horne, P. (1997), Sludge recycling to agricultural land; the environmental scientist’s perspective. Journal of the Institute of Water Engineering and Management, 11, April.
[40] CEC (European Commission) (2000), Working Document on Sludge, 2nd draft, 12/1/2000 ENV.E.3/LM, CEC, Brussels.
[41] Werther, J. and Ogada, T. (1999), Sewage sludge combustion. Progress in Energy and Combustion Science, 25(1), 55-116.#
[42] Ekins, P . (1999), European environmental taxes and charges: recent experience, issues and trends. Ecological Economics, 31, 39-62.
[43] van der Kleij, M., Dekker, R.H., Kersten, H. and Wit, J.A.W. (1991), Water management of the river Rhine: past, present and future, European Water Pollution Control, 1(1), 9- 15.
[44] French Environment Ministry (FEM) (1986), Studies of the Redon river and of phosphorus enrichment of Lake Geneva, Report of FEM Phosphorus – Redon sub- working group. Paris.
[45] Smith, S. (1997), Environmental Tax Design. In: T. O’Riordan (ed.) Ecotaxation, Earthscan, London.
[46] EUROSTAT (1992), Family Budgets: Comparative Tables Denmark, Greece, France, Ireland, Luxembourg, Netherlands. EUROSTAT, Luxembourg.
[47] EUROSTAT (1993), Family Budgets: Comparative Tables Belgium, Germany, Spain, Italy , Portugal, UK. EUROSTAT, Luxembourg.
[48] CFEGP (Commission de Finances, de l’Economie Générale et du Plan) (1999), La Taxe générale sur les activités polluantes et la politique de l’eau. Information report No. 1807, CFEGP, Paris.
[49] French Ministry of Finance (FMF) (2000), Taxe generale sur les activites polluantes (TGAP). Bulletin officiel de douanes, no. 6421, 4 April 2000.
[50] European Union Conciliation Committee (EUCC) (2000), Joint text approved by the Conciliation Committee: Directive 2000/ /EC of the European Parliament and of the Council of establishing a framework for Community action in the field of water policy, conciliation@europarl.eu.int refs. 1997/0067(COD) C5-0347/00 document, EU Parliament, Brussels.
[51] European Environment Agency (EEA) (1998), Europe’s Environment: the second assessment, EEA, Copenhagen.
[52] Hosper, S. (1998), Stable states, buffers and switches: an ecosystem approach to the restoration and management of shallow lakes in the Netherlands. Water Science and Technology, 37(3), 151-164.
[53] van der Molen, D., Portlieje, R. and Boers, P . (1998), Changes in sediment phosphorus as a result of eutrophcation and oligotrophication in Lake Veluwe, the Netherlands. Water Research, 12(11).
[54] Lang, C. and Reymond, O. (1996), Reversal of eutrophication in four Swiss lakes: evidence from oligochaete communities. Hydrobiologia, 334(1-3), 157-161.
[55] European Environment Agency (EEA) (1999), Environment in the European Union at the turn of the Century, EEA, Copenhagen.
[56] Key Note (1997), Household soaps & detergents: 1997 Key Note plus market report 11th ed. edited by Zoe Ratcliff, Hampton: Key Note Publications.
[57] Key Note (1999), Household soaps & detergents: 1999 Key Note plus market report 12th ed. edited by Zoe Ratcliff, Hampton: Key Note Publications.
[58] Chemical and Engineering News (2000), Rhodia expedites integration of Albright & Wilson, Chem Eng News, 162(21: May 24th), p.28.