How Do Italian Biotechnology Startups Survive?
Abstract: This study was carried out in order to better understand the economic and managerial characteristics of the biotechnology industry, which has taken hold in Italy at a much slower rate than in other industrialized countries. In particular, the specific aims of this paper are to analyse the business models of new Italian biopharmaceutical firms and to examine how these firms, with their different business models, successfully overcome the initial stage of starting up. On the basis of interviews conducted with sector experts and managers, we will conclude that the success factors behind the startups comprise a diverse range of distinct competencies that depend on the type of business model adopted.
Introduction
Biotechnology is one of the most significant emerging technologies and generates many applications in different fields, such as healthcare, agriculture, the food industry, fine chemistry and the environment.
The biotechnology industry was pioneered in the Unites States where small biotechnology firms were started up in the early 1980s; the development of the biotechnology industry, however, has not been uniform in all countries. In Europe, with the sole exception of the United Kingdom, the business of biotechnologies developed much later than in the United States due to the following main reasons [1, 2]:
- The problem of finding financing for these new firms (in particular the absence of a structured venture capital system)
- The limited protection guaranteed by the patents system
- The weak relationship between Academia and Industry, which complicates the knowledge transfer process
The fragmentation and specialization of research.
As in other European countries, the development of the biotechnology industry has taken hold in Italy only recently; in fact, even though there is a good level of scientific and technical competency, particularly in the pharmaceutical industry [3], the factors mentioned above have delayed growth.
In the pharmaceutical sector, in particular, biotechnology is doomed to undergo a revolution that will change the industry’s structure: on one hand a process of concentration of large firms has begun to take place and on the other hand new small biotechnology firms specialising in a particular discipline or research activity have been launched.
As a consequence, the biopharmaceutical sector is organized as a network made up of universities, public and private research centres, small biotechnology companies and large consolidated companies all working in collaboration rather than in competition.
The first aim of this paper is to analyse the different business models adopted by the companies that constitute this network; in identifying this taxonomy of business models, particular attention was paid both to the value chain activities of these companies and to the products and services they exchange with the market. We expected some of the critical factors for competition to depend on the type of business model adopted. The second aim of this paper is to analyse the following issue: for each of the business models identified, we have examined one biopharmaceutical startup firm in order to study the critical factors that have allowed it to survive and compete in this context. In other words, we are interested in examining how new biopharmaceutical firms with different business models successfully overcome the initial stage of starting up.
In order to identify the business models adopted by biotechnology companies, we first analysed the specialised literature [4, 5] and then confirmed the results obtained from the literature review by interviewing some sector experts (e.g. the head of the National Association for the Development of Biotechnology).
Concerning the second objective of the study, which was to analyse the success factors of biopharmaceutical firms, we conducted eight interviews both with the CEO of these firms and with their R&D managers. The main topics discussed regarded:
- The firm’s history: particular attention was given to the problem of finding financing The firm’s offering: type of products/services, characteristics of the pipeline, production strategy (internal manufacturing/outsourcing/licensing), type of distribution
- Market factors: nature of the market (local, national or international), position of the customer in the value chain, market breadth
- Internal capability factors: the source of internal competence was identified
- Competitive strategy factors: the position of the firms in the competitive arena was analysed
Innovative performance of the firm: trend of R&D investments, number of patents obtained, trend of employees, number of products/ processes/ services being employed.
The Business Models of Biopharmaceutical Companies
Biotechnology has contributed to a change in the pharmaceutical sector in both the internal processes and the external industrial structure [4]. Concerning the former, the use of biotechnology has modified the research methodology and the production processes.
Concerning the latter, biotechnology has changed the role played by pharmaceutical companies by creating a network of firms specialized in different activities along the value chain. Consequently, the pharmaceutical industry has taken a new configuration: while until the late eighties the sector was constituted by large vertically integrated companies, now it comprises a system of companies specialized in a particular stage of the innovative process, in a particular discipline, or in supplying services. The structure of this industry has become more complex and consequently different business models are adopted by biopharmaceutical firms. One of the aims of this paper is to suggest a taxonomy of these different business models on the basis of literature analysis [4,5] and the interviews conducted.
Five different business models were identified:
New biotechnology firms: typically carry out research activities until lead optimisation, and then license their outputs (i.e. drug candidates) to other firms
- Integrated firms: have a strong pipeline and carry out all the primary activities of the value chain, from target identification or lead optimisation to product commercialization. They thus cover the research and development phase, preclinical and clinical development, the post- approval phase, and production and commercialization activities
- Manufacturing companies: typically acquire the results of the research carried out by other companies and dedicate their efforts to the latter phases of the innovative process by carrying out engineering, production and commercialization activities
- Biotech suppliers: are firms that carry out the industrial development and production of biotech products for other firms. These companies use biotechnology in the production process in order to obtain biological products, such as monoclonal antibodies, cells and proteins, and supply them to other biotechnological companies. They develop production processes often in collaboration with customers and end their role with the supply of the product
Services firms: sell research services such as chemical synthesis, the study of cloning, and sequencing to drug-oriented companies that want to enhance their organisational competences. The startup capital required from this type of firm is smaller than that required by the previous business models.
Literature analysis enabled us to recognize the following business models: new biotechnology firms, integrated firms, manufacturing firms and service firms. We decided to exclude platform firms (also identified in the cited literature) because their core business is constituted by developing and commercializing technologies (i.e. physical devices and software tools) to support the R&D process behind new drugs. The business model represented by biotech suppliers was identified through the interviews that at the same time were also used in order to confirm the previous business models.
After identifying this taxonomy, we selected one Italian startup firm for each of the aforementioned business models with the aim of analysing the success factors which have allowed them to overcome the startup stage. The firms selected were the following:
- Company A, founded in 1998 from a previous industrial unit, is a new biotechnology firm (NBF) whose core business is focused on discovery and preclinical development stages. While this study was being carried out, the firm was involved in a merger process.
- Company B was founded in 1996 after a “management buyout” with the aim of becoming an integrated firm. It operates in all value chain activities, from the research stage to product manufacture and commercialization. When this study was being carried out, the firm had already built the production plant, but it still hadn’t set up a network for commercializing its own products (which were currently in the second clinical stage).
- Company C, founded in 1999, is a firm that carries out customized production for other companies. It uses biotechnology as a production methodology in order to develop biological products such as cells and monoclonal antibodies.
- Company D, founded in 2001, provides services (such as sequencing, diagnostic tests, training, etc.) in the field of diagnostics and research to firms belonging to different industries.
Manufacturing companies have not been considered in this analysis because in the database [6] available on the Italian biotechnology industry there are no start-up firms that belong to this type of business model in Italy.
The four firms considered in this study successfully overcame the startup stage as the most used performance indexes [7, 8, 9] highlighted:
- Increase in R&D investments
- Increase in the number of employees
- Number of patents obtained
- Number of products/processes/services being developed.
At this point, it is interesting to identify the success factors that have allowed these firms to overcome the startup stage successfully; in this way, a connection is made between success factors and business models.
The Success Factors of Biopharmaceutical Startup Firms
The resource-based view represents the theoretical framework that has been adopted in this study in order to identify success factors; particular attention was then given to the set of resources and competences developed by the firms. In particular, resources were defined as stocks of available factors that are owned or controlled by the firm [10], while competence was defined as “the collective knowledge of an organization”, and in particular, “the capacity for the team of resources to perform some tasks or activities” [11].
While analyzing competences, it emerged that their nature changed according to the type of business models adopted.
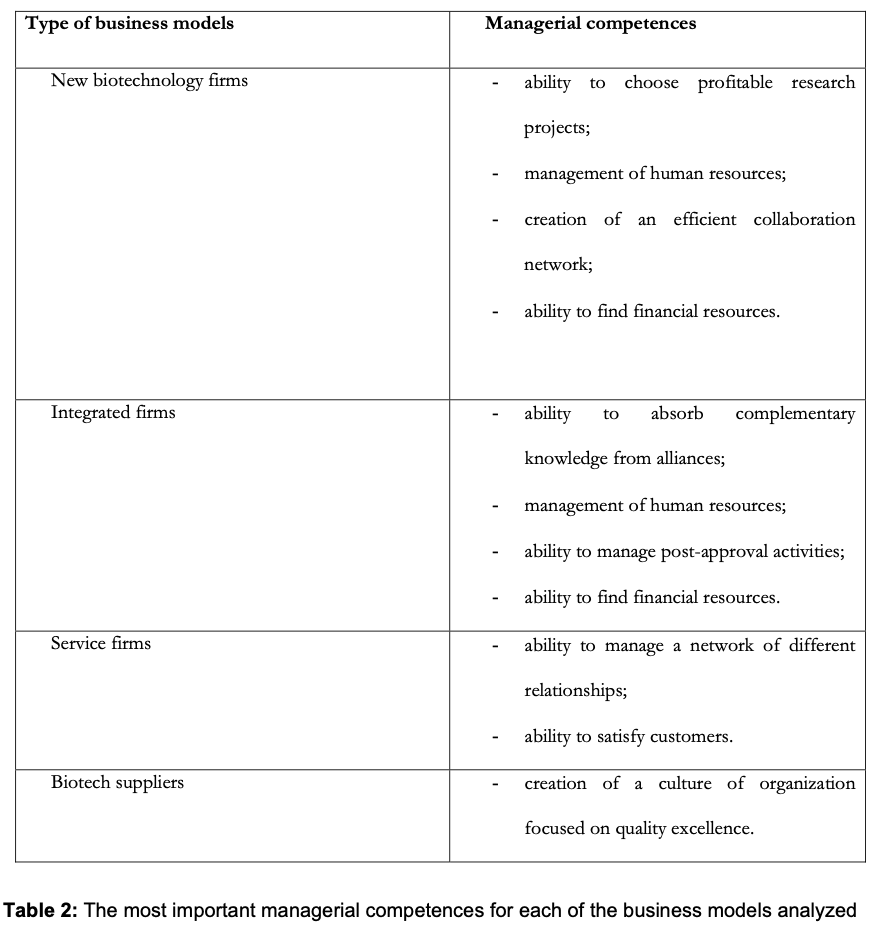
In case A, although technical-scientific know- how is very important for the development of new products, managerial competences also play a fundamental role in the success of the startup; indeed, the study identified the following as the most important managerial skills:
- Ability to choose profitable research projects
- Management of human resources
- Creation of an efficient collaboration network
- Ability to find financial resources.
While this study was being carried out, Firm B was in a similar situation to Firm A because it carried out research and clinical development, even though it had built the production plant and was setting up a sales network. In this case, technical know-how (mainly combinatorial chemistry and high throughput screening) and managerial competences are the most important success factors for firm B as well. Concerning managerial competences, the skills that emerged from the analysis are the following:
- Ability to absorb complementary knowledge from alliances
- Management of human resources
- Ability to manage post-approval activities
- Ability to find financial resources.
The literature [12, 13] sustains that production and marketing skills are fundamental for the success of integrated biopharmaceutical firms, but at this point these skills cannot be examined because of the young age of the companies.
Firm C focused on quality excellence as the fundamental guiding force behind all these activities. From the startup phase, the management’s efforts were geared towards the implementation of a set of procedures and practices (e.g. the good manufacturing practices system) that ensure the manufacture of high quality products. The efforts of this firm’s management were devoted to the creation of a culture of organization focused on quality excellence and many activities (weekly meetings, training programs, team building) were implemented for this purpose.
Firm D overcame the startup phase by developing a tight network of relationships with many different external partners, which allowed the firm to widen its range of services, increase its technical knowledge and strengthen its customer portfolio. The firm’s management guided its activities to overcome its present confines in order to create a new organizational configuration: a network of firms, with units comprising different organizations, such as research institutes, customers, diagnostics laboratories, universities, etc. Even the attention given to customers emerged as a critical element for the success of the startup.
Finally, it is important to underline that all the firms considered were founded by people who had previously worked for multinational firms and thus had substantial technical and managerial experience in this industry. In all the firms analysed, many different organizational processes have been activated by the management in order to transfer their tacit knowledge to new company employees.
Lessons
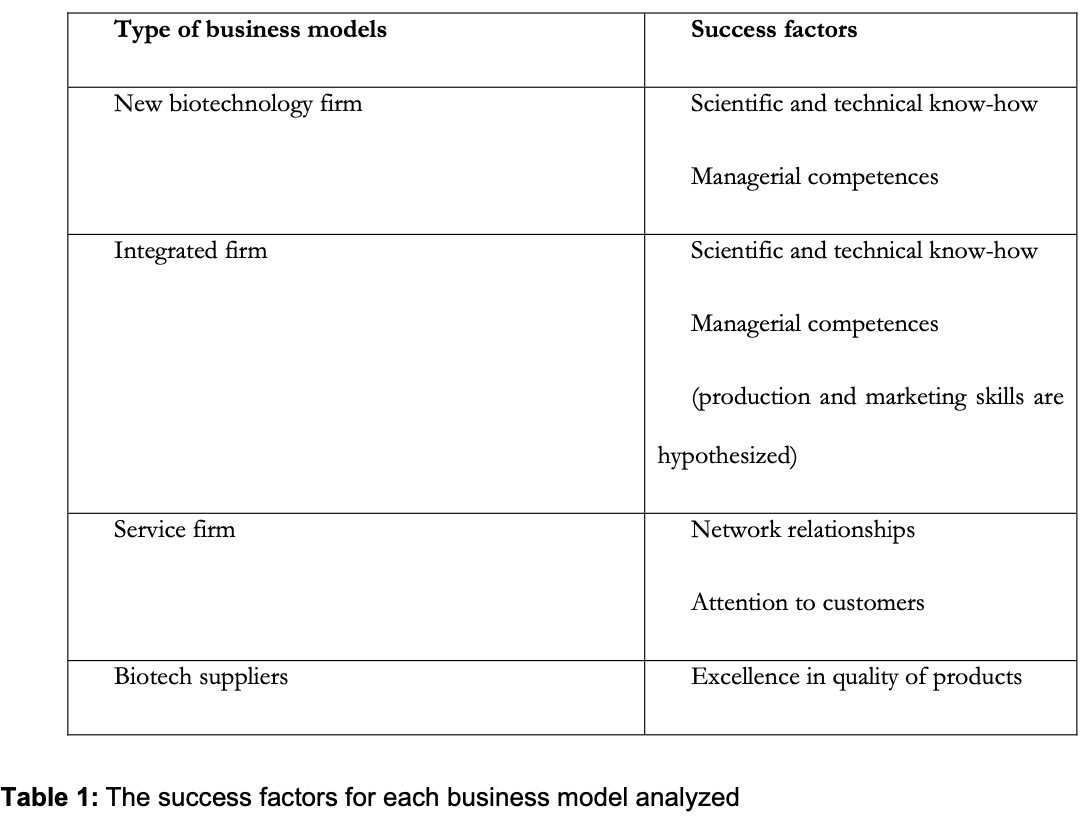
This study on the one hand identifies a taxonomy of business models in the biopharmaceutical industry and on the other hand shows how four biotechnology startups overcame the startup stage successfully. As illustrated above, startup success factors encompass a diverse range of distinct competences according to the type of business models adopted. Table 1 shows the success factors associated with each business model:
In other words, this case analysis illustrates that the success elements are connected with the firm’s strategic value chain activities.
In the case of new biotechnology firms, where research activities play a critical role in success, specific technical and scientific know-how (e.g. in target identification/validation, or in lead identification/optimization) is fundamental.
In the case of integrated firms, where different value chain activities can play a critical role in success, both technical and scientific know-how, as well as an ability to perform approval activities and production and marketing competences, are important.
In the case of services firms, where the most important activities are located at the end of the value chain, the ability both to satisfy customers and to establish many relationships with different organizations are strategic for success.
Finally in the case of firms that carry out industrial development and production for other firms, the ability to guarantee excellence in final- product quality is the most important element for the success of a firm.
It is interesting to point out that, as Table 2 shows managerial competences played a fundamental role in the success of all the startups examined regardless of the type of business models adopted; management of human resources, the ability to build and manage a network and implementation of the quality system are some examples of the managerial competences required. In all the cases analysed, these competences are generated by the founders who had accrued significant experience in this industry.
The results of the study confirm the recent literature on this topic. In fact many studies [14, 15, 8] have maintained the importance of inter- organizational relationships, e.g. networks that generate new knowledge and create competitive advantage. Even the role of technical competence in the success of biopharmaceutical firms has long been recognized by many Authors [12, 16, 13]; Pisano [17] in particular stressed the role of the production process to guarantee a quality product. If, on one hand, the role of technical competence is fundamental, on the other hand managerial competence can also play a significant role in the success of biotechnology firms [18, 2].
The study is original in that it concerns the identification of different strategic competences according to the type of business model adopted.
The suggestion emerging from this study is very important in particular for academic startups, where managerial skills are usually lacking in the scientific staff working at the university; for this reason the assistance given by technology transfer offices or other local authorities, in terms of organizational and financial support, could be very important.
In particular in Italy, where there are very few university spin-offs in the field of biotechnology, an interesting solution to joint scientific and managerial competencies could be the launch of university startups where the founders are a team of both academic scientists and industry managers.
Finally, a look at the study conducted by Weisenfeld-Schenk [19], who proposed a typology of biotechnology firms based on the strategic types described by Miles & Snow, reveals some similarities and suggestions for further research. The Weisenfeld-Schenk study classifies biotechnology firms into three main groups: the first group is constituted by companies that “pursue process improvements and are lowest on new product development”; the second group encompasses companies that are mainly devoted to basic research instead of applications engineering; and the third group is the closest to the market and between the first and second groups. Looking at this classification, we can see, for example, that cluster 2 described by Weisenfeld-Schenk seems to share some common points with the new biotechnology firms presented in our study. Further research could then investigate the strategic behaviour of biotechnology firms through a quantitative analysis that takes into consideration both the strategic types suggested by Weisenfeld-Schenk and our own findings about business models.
References
[1] Orsenigo, L. (2001), The (failed) development of biotechnology cluster: the case of Lombardy, Small Business Economics, 17, 77-92.
[2] Hall, L.A, Bagchi-Sen, S (2002), A study of R&D, innovation, and business performance in the Canadian biotechnology industry, Technovation, 22, 231-244.
[3] Gambardella, A (1996), Prospettive e proposte per uno sviluppo della R&S nell’industria operante in Italia nella biotecnologia farmaceutica, Assobiotec Federchimica.
[4] Chiaroni, D., Chiesa, V., Toletti, G. (2003), Business models in the bio-pharmaceutical industry, The R&D management Conference, Manchester, 7-9 july.
[5] Bigliardi, B., Nosella, A., Verbano, C., (2005), Business models in Italian biotechnology industry: a quantitative analysis, Technovation, Volume 25, Issue 11, November, 1299-1306.
[6] Nosella, A., Petroni, G., Verbano, C. (2004), Characteristics of the Italian Biotechnology Industry and new business models: the initial results of an Empirical Study”, Technovation, vol.25, n.8, pp.841-855.
[7] Zahra, S. A., George, G. (1999), Manufacturing strategy and new venture performance: a comparison of independent and corporate ventures in biotechnology industry, Journal of high technology management research, 10, 313-345.
[8] Baum, J.A.C., Calabrese, T., Silverman, B.S. (2000), Don’t go it alone: alliance network composition and startups’ performance in Canadian biotechnology, Strategic management journal, 21, 267-294.
[9] Garcia, C.Q., Velasco, C.A.B. (2002), Co- opetition and performance: evidence from European biotechnology industry, II annual conference of Euram on innovative research in management, Stockholm (Sweden), may 9-11.
[10 ] Amit, R., Schoemaker, P.J.H. (1993), Strategic assets and organizational rent, Strategic management journal, 14, 33-46.
[11] Grant, R.M. (1991), The resource based theory of competitive advantage: implications for strategy formulation,California management review, spring, 33.
[12] Henderson, R., Cockburn, I. (1994), Measuring competence? Exploring firms effects in pharmaceutical research, Strategic management journal, 15, 63-84.
[13] DeCarolis, D.M. (2003), Competences and imitability in the pharmaceutical industry; an analysis of their relationship with firm performance, Journal of management, 29, 27-50.
[14] Powell, W.W. (1998), Learning from collaborations: knowledge and networks in the biotechnology and pharmaceutical industries, California management review, 40, 228-240.
[15]Decarolis, D.M., Deeds, D., L. (1999), The impact of stocks and flows of organizational knowledge on firm performance: an empirical investigation of the biotechnology industry, Strategic management journal, 20, 953-968.
[16] Quelin, B. (2000), Core competencies, R&D, management and partnership, European management journal, 5, 476-487.
[17] Pisano, G.P. (1997), The development factory: unlocking the potential of process innovation, Harvard business school press, Boston, Massachusetts.
[18] Woicheshyn, Hartell (1996), Strategies and performances of Canadian biotechnology firms: an empirical investigation, vol 16, n 5, pp 231-243.
[19] Weisenfeld-Schenk, U. (1994), Technology strategies and the Miles & Snow typology: a study of the biotechnology industry, R&D management, 24, 57-64.