Development of an effective outsourcing strategy for toxicological studies in the chemical industry
Abstract
The chemical industry has been put under considerable time pressure by the European Community Regulation REACH (Registration, Evaluation, and Authorization of Chemicals). The work outlined here has been developed at the BASF SE’s Experimental Toxicology and Ecology Unit with the objective of promoting a faster reaction to the testing demand generated by the new legislation. A considerable increase in forecasted demand for tests has created the necessity to increase the Toxicology Unit’s outsourcing activities. The first goal was to optimize the selection and management process of Contract Research Organizations (CROs), so that toxicological studies can be performed with minimal risk while maximizing quality and cost advantage. A second objective was to develop performance measurement system in form of a balanced score card to evaluate contracting efficiency by monitoring major drivers in the outsourcing process to ensure the alignment between strategic objectives and actual performance.
1 Introduction
The BASF Experimental Toxicology and Ecology in Ludwigshafen is a global internal service provider that performs toxicological studies for chemical and agrochemical substances as well as ecological studies for chemicals.
Because REACH came into force in June 2007, the demand for registration of all chemical substances produced in or imported to the European Union is having a considerable impact on the BASF SE business units, forcing them to comply with such new registration requirements in a short period of time, not only in terms of workload, demanding new structures and processes, but also demands a substantial financial investment in registration of substances.
This paper is a hands-on work, and discusses how concepts and theoretical approaches are applied under real conditions and how diverse tools and methods can be used for strategic decision-making. Companies are constantly facing new challenges that come from market competition and the supply chain as well as political or economic environments, such as regulations. These force organisations to change their operations and processes and to develop new strategies to survive or grow in a changing environment.
The first method to be used here is the Strategic Management Model (Wheelenand Hunger, 2005), which is the guideline for the challenges assessment that BASF Experimental Toxicology and Ecology in following named “Toxicology Unit” has been facing and offers a structured form to develop a solution. The author Grönroos (1990) defined the service concept, which was applied to the Toxicology Unit’s strategic role and to explain how it can contribute to improve the outsourcing activity. Literature researches of outsourcing related themes lead to the conclusion that one of the first industries that operate outsourcing is the information technology industry. For this reason, most examples of best practices in this field have come from authors that have been studying outsourcing in this industry (see references).
The authors Poweretal. (2008) propose a method to evaluate the development, or maturity, level of outsourcing strategies that helped identify the improvement points in the current strategy as well as risks pertinent to outsourcing(Carvusgil etal., 2008). Amodel from the US General Accounting Office (2001) gave inputs to define the outsourcing life cycle for toxicological studies. Besides, experiences from the pharmaceutical industry published on books (such as Winter and Baguley, 2006) and on this journal along with inputs from an external consultant were major drivers to determine strategic guidelines, to improve the workflow, to enhance the supplier’s selection and evaluation and to implement an effective study monitoring process.
As elucidated by the Strategic Management Model, an important part of any strategy is the monitoring of the results obtained with the strategic plan. The controlling system ensures that the strategy deliveries the expected results or, in this case, that the contracting and management of service providers are being conducted efficiently and in alignment with the strategic objectives.
To address this point, the author Cullen (2009) has provided her experience with performance measurement of contracts. The contracts core card as defined by her is a balanced score card developed for the context of contract management. This paper applies it to monitor the outsourcing performance by evaluating the activity from different perspectives. It aims to be a feedback mechanism to the entire life cycle, ensuring the strategic objectives achievement and the process continuous update.
In the end, some of the trade-offs in the decisions involving outsourcing will be discussed and the authors will expose their conclusion and the possible next steps of this work.
2 Current outsourcing strategy of Toxicology Unit and its results
The contract management team is responsible for requesting and evaluating quotations, selecting and contracting service providers, so called CROs (contract research organisations), and ensuring compliance with international and BASF SE standards, e.g. Animal elfare Policy, OECD guidelines for testing, GLP (Good Laboratory Practice ) requirements, and legal and purchasing guidelines.
An active system for study portfolio management has been implemented with the objective of managing demand fluctuation through identifying the appropriate types of study for outsourcing in order to reserve internal capacity for higher tier studies.
- The studies were evaluated according to three criteria: the ratio between market prices and internal cost
- the importance for the BASF Group, i.e. the existence of intellectual property issues (IP) on substances, a critical aspect for testing new developments
- the number of suppliers (CROs) available for each expertise field.
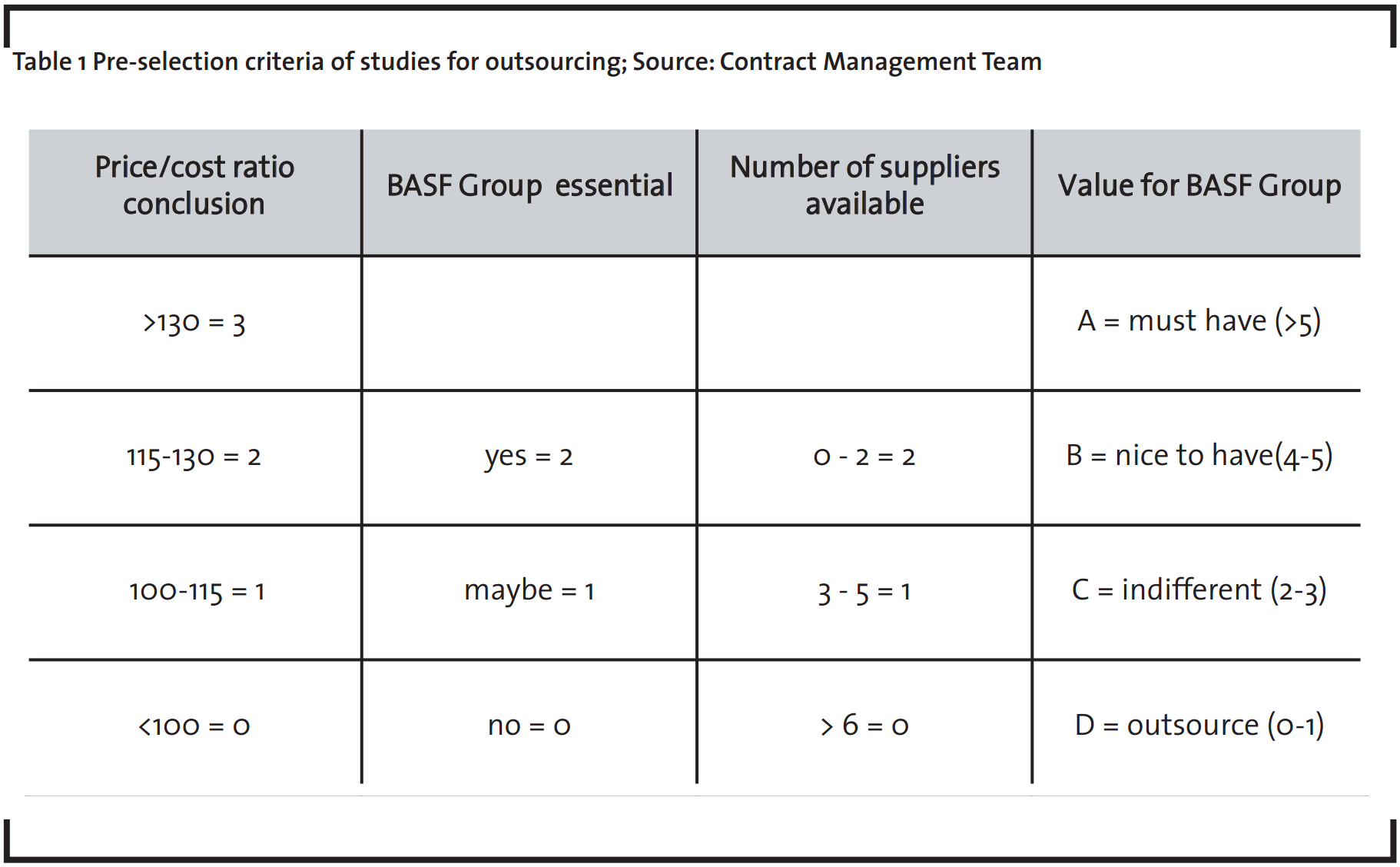
On regular meetings between the top management and technical experts of the Toxicology Unit, each parameter receives points, according to the criteria explained above. The subjectivity of the whole evaluation is minimized by the definition of clear criteria, i.e. the number of available suppliers in the respective study type field. The sum of the given points for the different criteria leads to the classification of the studies in four categories (A, B, C and D), which corresponds to the value for the BASF SE showed in Table 1. The list of classified studies has been named Priority List of Studies.
- Studies receiving more than 5 points are highly critical and are performed in-house. They represent the unique strengths of BASF SE, like screening tests, metabolomics and special repeated-dose studies
- Studies receiving 4 or 5 points are studies of high interest for the Group and are performed preferably in-house. This category includes studies where BASF SE is competitive in price, offers superior experience and there are few alternative suppliers available. Particular attention must be paid to confidentiality agreements
- Studies receiving 2 or 3 points are standard studies and have a moderate price/performance ratio and there are plenty of suppliers available. The decision of contracting to a service provider depends on internal capacity availability
- Studies receiving less than 2 points reflect a low interest for the BASF SE. They have a low price/performance ratio and/or are expected to be replaced by alternative methods. Here an active outsourcing strategy will be applied.
The outsourcing group’s responsibilities also include:
- Communication with internal stakeholders
- Communication with CROs
- Providing specific project information regarding, for example, deadlines
- Provision of test substance and test substance information
- Monitoring of studies; this is the main mechanism to ensure the desired quality level and compliance. The study type is the determining factor for choosing the appropriate study monitor with the appropriate expertise.
After a study is concluded with the final report delivery, the contract management team collects feedback about the CRO and informs the sponsor about its performance regarding the specific study.
The critical elements of the outsourcing process are:
- Suppliers’ selection and evaluation
- Study monitoring
- Compliance with the BASF SE standards
- Monitoring of contract obligations
- Management resources assurance
- Communication between the involved parties.
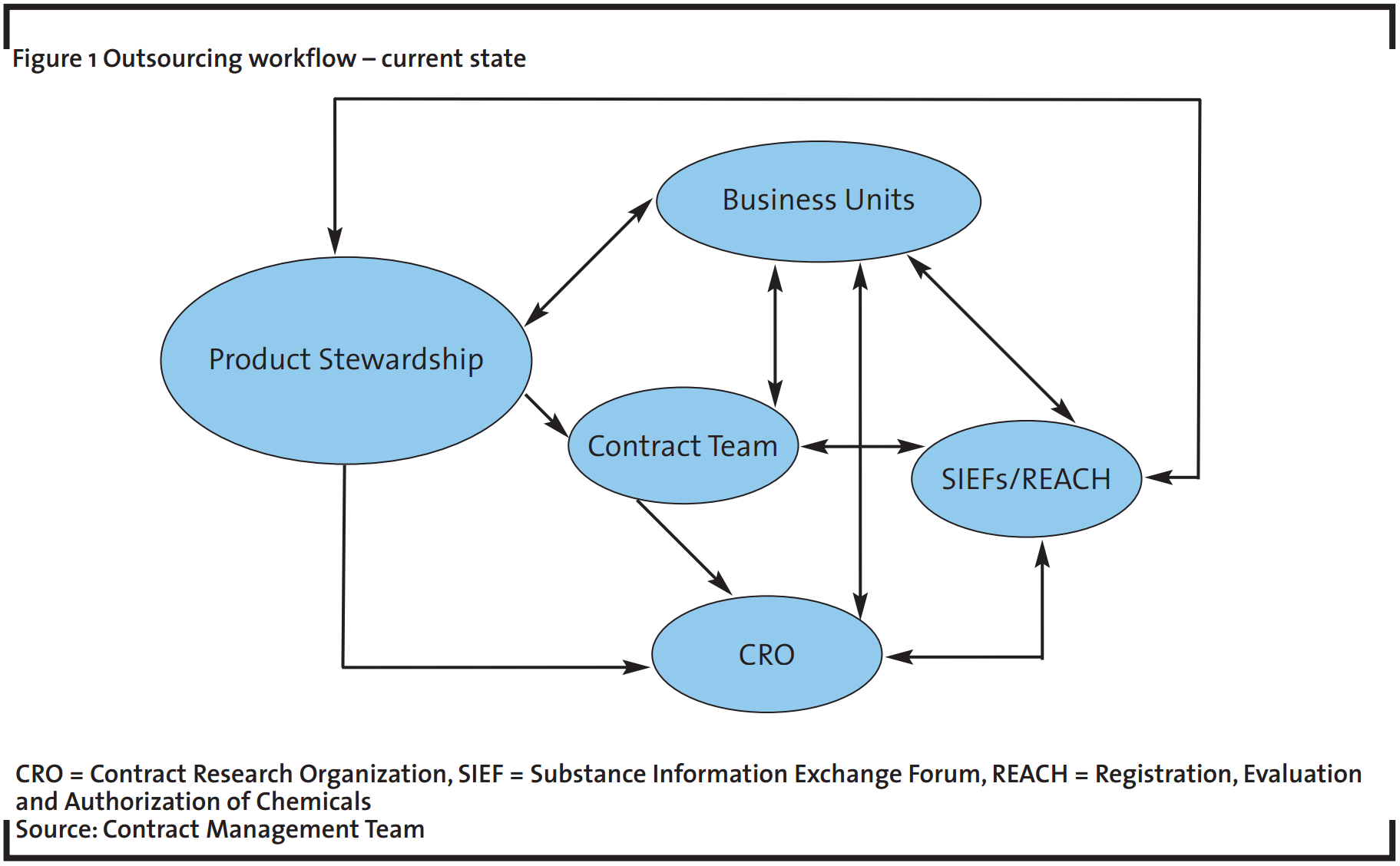
In the past the contract management team, sponsor (business units) and product stewardship staff have all requested quotations independent from each other and placed work with CROs without sufficient coordination. Figure 1 shows the previous outsourcing workflow.
The outsourcing process shown in Figure 1 has added value to the BASF SE by:
- Providing extra capacity without expanding internal infrastructure and fixed cost: addressing internal demands without binding to a long-term investment (in assets and, mainly, in personal)
- Offering an external benchmarking opportunity to assess internal efficiency and prices
- Providing complementary technical capabilities: technological competencies in special niches that differ from those of the BASF SE’s Experimental Toxicology and Ecology Unit
- Covering other geographic areas: studies required by regional authorities that must be conducted locally
- Reducing costs: as some services, e.g. acute studies, may be performed better and/or cheaper by CROs and this frees up capacity at the BASF SE’s Experimental Toxicology and Ecology for performing studies that offer a better cost margin or are critical to the organisation
The contract management strategy will be critical in maintaining the BASF SE’s competitive advantage by outsourcing services that do not add technological or economic value to the Group. By making use of the core competences of external service providers, it allows a more flexible and efficient approach towards internal capacity management.
Additionally, decision-making based on the Priority List safeguards the BASF SE’s interests in intellectual property issues on substances, regarding the study method and test substance information. Different suppliers’ selection and contracting approaches are taken depending on the study classification. In the case of categories C and D, the focus is set on quality and costs while for A and B the major concern lies in maintaining quality and confidentiality in the external environment.
The previous strategy was adequate however it could not be sustainable or efficient in a future scenario of increasing demand for studies.
According to the Outsourcing Management Maturity Model (Power, et al., 2008), organizations go through different development stages during an outsourcing program. The authors’ experience has proved that the faster an organization learns from its mistakes and changes the intrinsic behaviours that cause the mistakes, the better it is positioned to be successful in the future.
In the first stage of its outsourcing development, the BASF SE’s Experimental Toxicology and Ecology Unit has put in place many processes to facilitate outsourcing. The contract team has been formed, strategic objectives have been set and some processes such as a selection procedure, contract facilitation through framework agreements, invoice management and controlling and the administration of test substances have been established.
However, to deal successfully with the scenario of increasing demand, the strategy should be enhanced based on the lessons learned so far and by applying industry best practices.
While the strategic assessment has been conducted and some parts of the process defined, the whole lifecycle lacks a complete integration. Some issues have become critical to a faster reaction time, such as the previous workflow, supplier selection and evaluation processes.
3 Enhancing the outsourcing activity
3.1 Establishing the service vision
A new organizational structure for contract management was established in the middle of 2010. The establishment of the group’s service vision helps elucidate its role within the Toxicology Unit regarding outsourcing activities, as well as to provide orientation to the development of an outsourcing strategy and to facilitate communication of its objectives. The service concept design has also the potential of reducing double work, aligning objectives and stimulating cooperation between stakeholders.
The starting point is the BASF SE’s expectation of the Toxicology Unit. This issue has been addressed by the Toxicology Unit’s management by involving also other main stakeholders like business units and corporate management. This research shows that the Unit is expected to:
- Provide results in time to meet REACH deadlines;
- Take responsibility for guaranteeing compliance of outsourced studies, e.g. regarding Animal Welfare and GLP;
- Take responsibility for guaranteeing compatibility of study and report quality with established standards.
According to the author Christian Grönroos (1990), the definition of service may depend on the way the customers or clients interact with the provider when the client receives the study final reports and during the process of production itself – the client is an integral part of the outcome production; he participates actively in how, what and when the study is to be conducted.
BASF SE’s Toxicology Unit’s mission concept will be used to determine in which markets it should operate and which kind of services it should provide. The basic material for a service concept development is market research, conducted through a series of interviews with the product stewardship and the business units. Based on this definition, the service vision of the Service Group aims to:
- Understand the clients’ needs, how they perceive value from the BASF SE’s Experimental Toxicology and Ecology Unit and how to meet those needs
- Guarantee that studies, both in- and outsourced, are conducted in compliance with Animal Welfare, GLP and contractual obligations Provide infrastructure to the BASF SE’s
- Toxicology Unit to conduct and archive studies internally
- Manage in- and outsourcing activities, which means:
- Providing infrastructure for conducting studies internally and externally
- Being a central point for contracting studies for internal or external clients
- Being a central point for selection, monitoring and evaluation of suppliers and contracts.
The following section is dedicated to the outsourcing process itself, identifying the outsourcing lifecycle and establishing the general guidelines for outsourcing to enable a smoother communication among the stakeholders and a more efficient process.
3.2 Identifying the outsourcing lifecycle for toxicological studies
Although outsourcing/off-shoring has the potential to increase the available capacity and increase flexibility at an international level, it also involves many risks, pointed out by experts in global outsourcing (Cavusgil et al., 2008 and Power et al., 2008). After studying many engagements, the authors have concluded that the main decisions to mitigate such risks involve defining the strategic outsourcing objectives, selecting the suppliers based on multiple criteria, investing in supplier development and collaboration as well as safeguarding the sponsor’s interests.
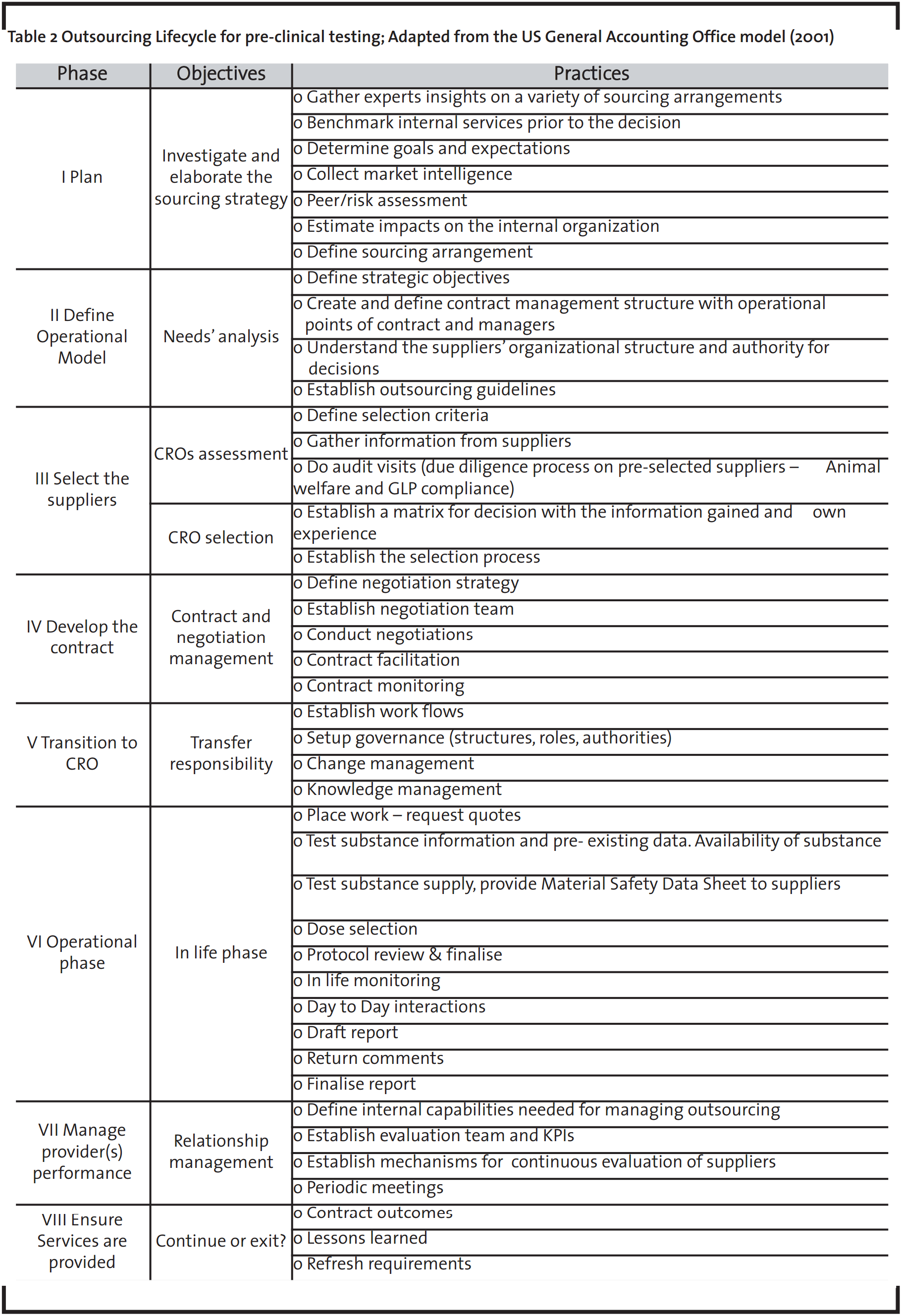
The outsourcing lifecycle framework is a tool that provides a step-by-step guide, integrating the strategic and the operational level of outsourcing objectives and results. Table 2 has been developed based on an outsourcing lifecycle model provided by the US General Accounting Office (2001). It shows the identified outsourcing phases for the toxicological studies as well as the major practices associated with each stage. This framework allows the visualisation of all the activities included in the toxicological services outsourcing process. Besides, it can form the basis for identifying the optimization points in the strategy. The focus of this paper is to:
- 1. Establish the outsourcing guidelines: communicate the parameters that are important for its success
- Improve CRO selection through clear and multiple criteria and the process itself
- Establish supplier management: evaluate and monitor performance to develop relationships further
- Establish some key performance indicators (KPI) for performance measurement.
3.3 Establishing the outsourcing model
The strategic decisions in outsourcing have been stressed in the referenced literature as the most important ones, forming the starting point to determine the outsourcing arrangement and to identify potential suppliers. Some aspects have to be considered in these decisions, like the study complexity and its operational impact as well as the strategic importance for the business. To guide this assessment, a recent report published in the Journal of Business Chemistry (Festel et al., May 2010) showed the different levels of cooperation models used in the pharmaceutical industry, which are similar to the toxicological studies in the chemical industry.
According to this report, the pharmaceutical industry invests much management time and capacity in choosing appropriate service providers to achieve goal congruence. Inspections of the suppliers’ facilities, quality, best practices, trained staff and certified processes are crucial in the selection process, as well as the assessment of their financial stability.
The principles of risks mitigation (Cavusgil et al., 2008) and the outsourcing model are the basis for establishing guidelines for an outsourcing strategy. The following aspects have been identified as critical for the success of the strategy and therefore should be followed by the whole company:
- Animal Welfare compliance by suppliers is a long-term critical aspect
- Adherence to all agreed and established outsourcing processes by stakeholders
- Observance of stakeholders’ accountabilities
- Compliance with a clear accreditation process
- Use of preferred and approved CROs only, at a global level considering regional needs
- Regional placement of work is allowed only with accredited CROs and through a process established by the global contract management team
- Establishment of internal contact points to each project
- Definition of governance structures in the process
3.4 Establishing a new management approach
Within large organizations, an unstructured and inefficient workflow may cause problems for the outsourcing management. For example:
- Poor Animal Welfare compliance
- Loss of control over the number and type of tested substances, the suppliers used and the observance of compliance and legal requirements
- Difficult assessment of quality differences and performance
- Different approaches regarding purchasing and legal aspects
- Difficulty in reporting to central controlling
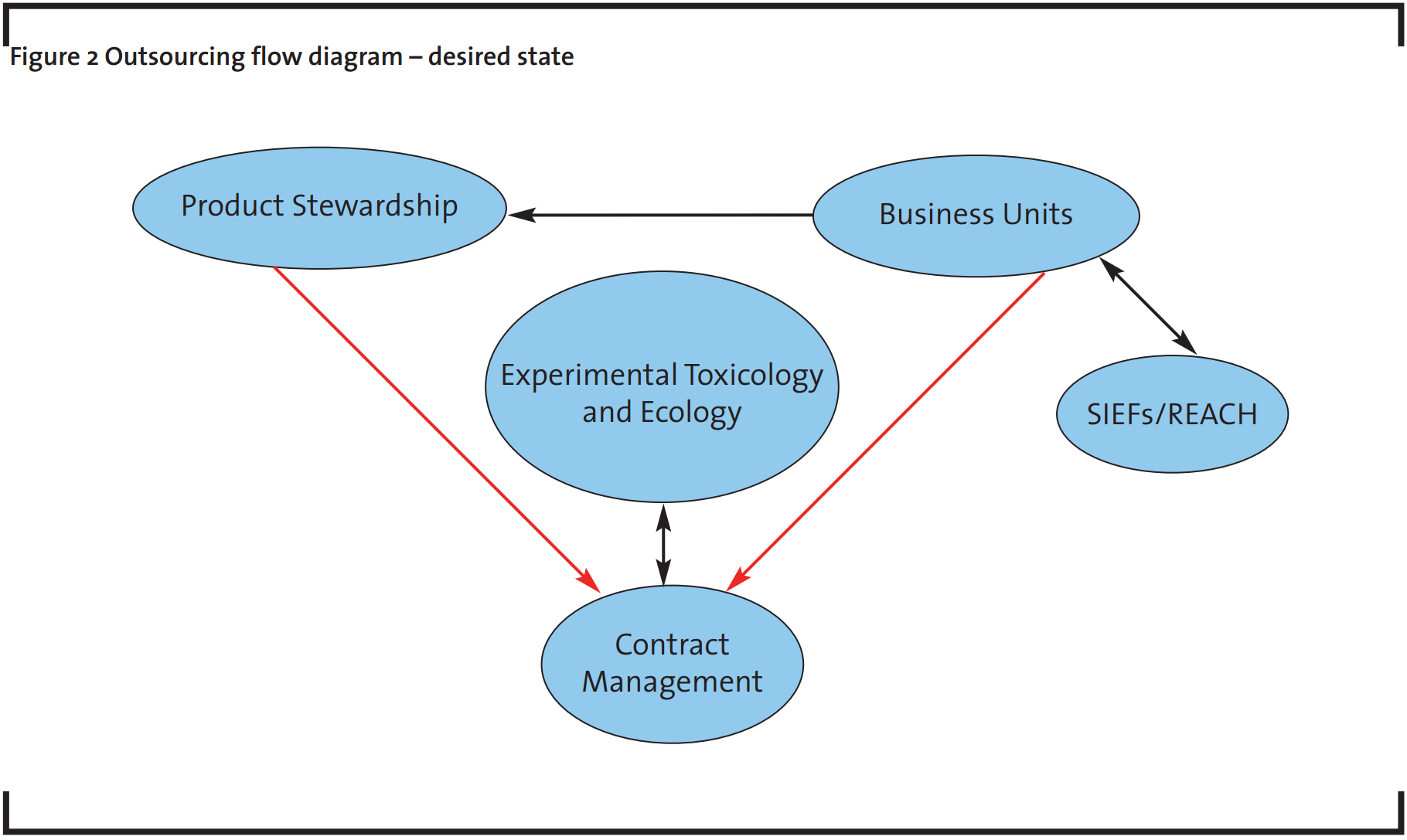
There is therefore a high probability that the overall relationship cost, in terms of time, money and resources, will outweigh the potential benefits of working with the CROs. For this reason, the relationship should evolve to a more structured one, as shown in Figure 2. A more centralized outsourcing workflow will help minimize the risks identified above and will enable the organisation to achieve its strategic objectives. The contract management team will act as a central contact point for outsourcing activities of toxicological studies at a global level within the company.
The previous relationship with suppliers, where many different members of internal staff talk to other members of external staff without prior coordination can be seen in Figure 1. There is a requirement to move to a structured relationship, as illustrated in Figure 2 (Winter and Baguley, 2006). The contract management team will coordinate the interactions between technical experts, purchasing, legal, etc. to optimize the information flow, allowing the experimental unit to concentrate on performing studies and to focus on the assessment of complicated cases that might arise on the suppliers’ side. The key goal is to gain efficiency through the structure alignment with suppliers and internal stakeholders.
An outsourcing management group must take responsibility for reporting every animal study to the relevant authorities according to the BASF SE Animal Welfare policy and rules for reporting procedure for animal studies (see Appendix I). A mechanism to guarantee this compliance is essential. Through this newly structured workflow the contract management team coordinates and manages the information about testing and ensures all company compliances, bringing some additional benefits:
- Compliance assurance with legal and procurement guidelines
- More transparency of the outsourcing process
- Contract facilitation
- Increase in supplier management effectiveness
- Facilitation of the establishment of a global outsourcing team
3.5 Improving the CROs selection process
Similar to a marriage, the supplier selection strategy should be designed to lead to a longterm, mutually beneficial relationship. Selecting the right supplier for each study type or technical discipline is essential to maximize the benefits and minimize the risks and costs associated with contracting out.
After having chosen a multisourcing model, i.e. the use of more than one CRO (Oshri et al., 2009), the next step will be to choose the criteria to select suppliers, considering the critical criteria to ensure the desired service level for the sponsor. It also represents a potential opportunity for the development of a sustainable supply chain management, as there is growing concern from the public about how companies are dealing with social standards and obligations such as animal testing, as reported in an article in the Journal of Business Chemistry (Peukert and Sahr, May 2010). In this sense, a sustainable procurement has to include, among other factors:
- Clear standards
- Selection, evaluation and control of suppliers according to these standards and expectations
- Appropriate and clear penalty
- Global coverage
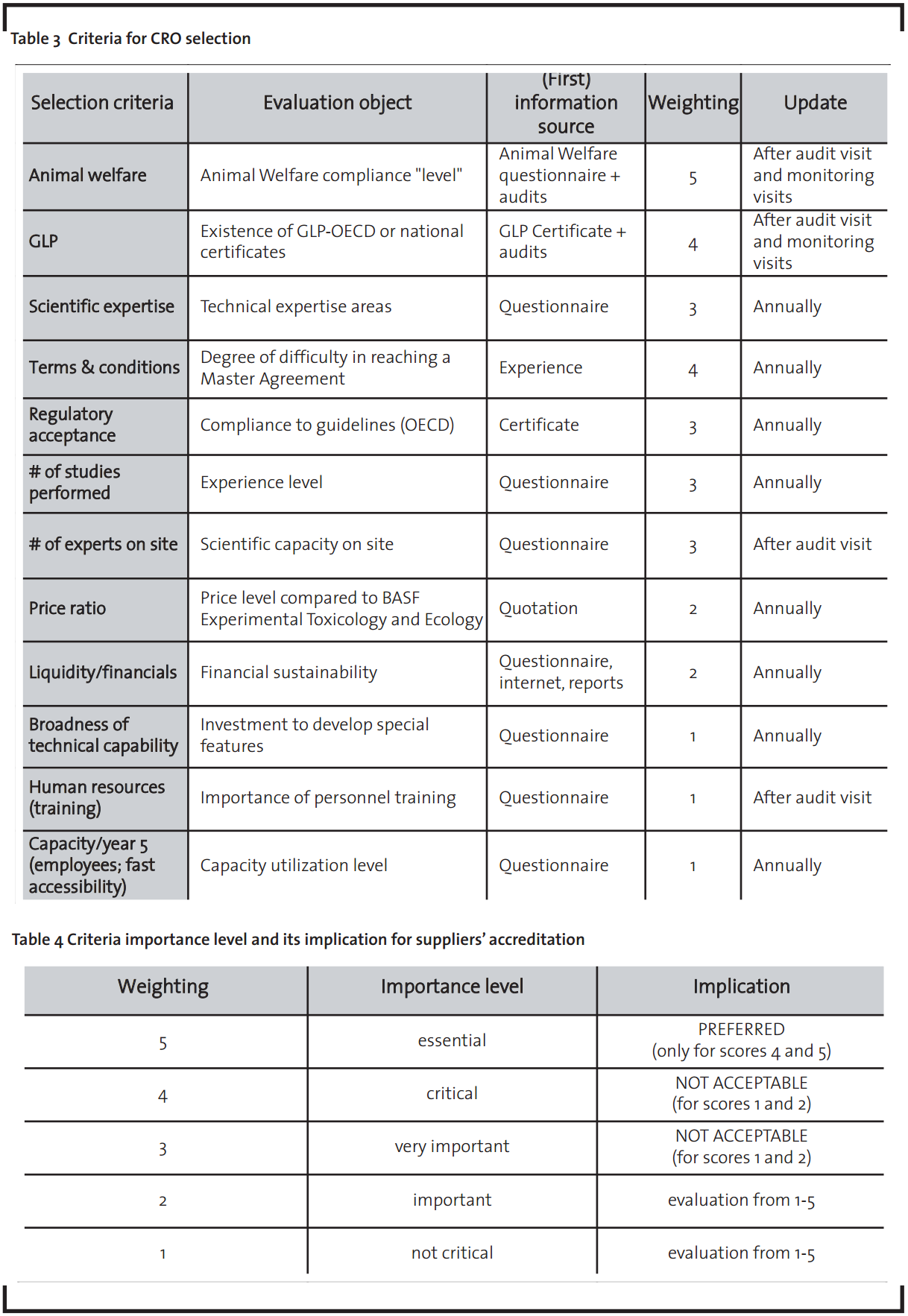
Selection criteria have be set to evaluate the suppliers’ competencies in delivering the services, transforming (supplier’s ability to deliver improved service both in terms of quality and cost) and building a relationship (also called “easy-to-work” competency, it includes the supplier’s willingness and ability to design its business model to the values, goals and needs of the customer) (Oshri et al., 2009). Based on the risk mitigation principle, these criteria, determined by the technical experts and the business specialists from the Toxicology Unit, form the technical and non-technical reference for the evaluation of the suppliers’ competitiveness, technical capabilities and capacities, investment plans and robustness from a long-term perspective.
A questionnaire, based on the criteria shown in Table 3, was developed to deliver information in four areas (Appendix II gives the main topics). CROs completed it with their data regarding scientific expertise and experience, financial development and projections, human resources management approach, and company market focus. The data will flow to a supplier matrix and will generate a supplier ranking that will form the basis for selecting companies that will be audited for Animal Welfare and GLP compliance, along with technical competence evaluation, a procedure that assures a transparent and sustainable outsourcing process, saving valuable time and money.
The questionnaire and Animal Welfare checklist improve the quality of the collected information and acts as a preparation for an audit visit. The audit of selected CROs (considering, for example, expertise, location and recommendations) is to be conducted by a team, normally comprising an internal technical expert (or experts), the Animal Welfare specialist (or officer) and a member of the contract management team (responsible for business criteria).
After the audit visit, scores for Animal Welfare and GLP compliance will be assigned to the supplier and determine if it can be accredited. The scoring system works according to a punctuation principle: the contract management team establishes to each criterion receives a weight between one and five. The weighting value of each criterion represents an objective form of quantification of its importance level in achieving the desired effectiveness of the outsourcing activity. As shown in Table 4 below, essential aspects for the outsourcing success receive the higher weight value 5. A regular revision of this weighting system ensures that the contracting of suppliers is being conducted according to the risk mitigation principle mentioned above.
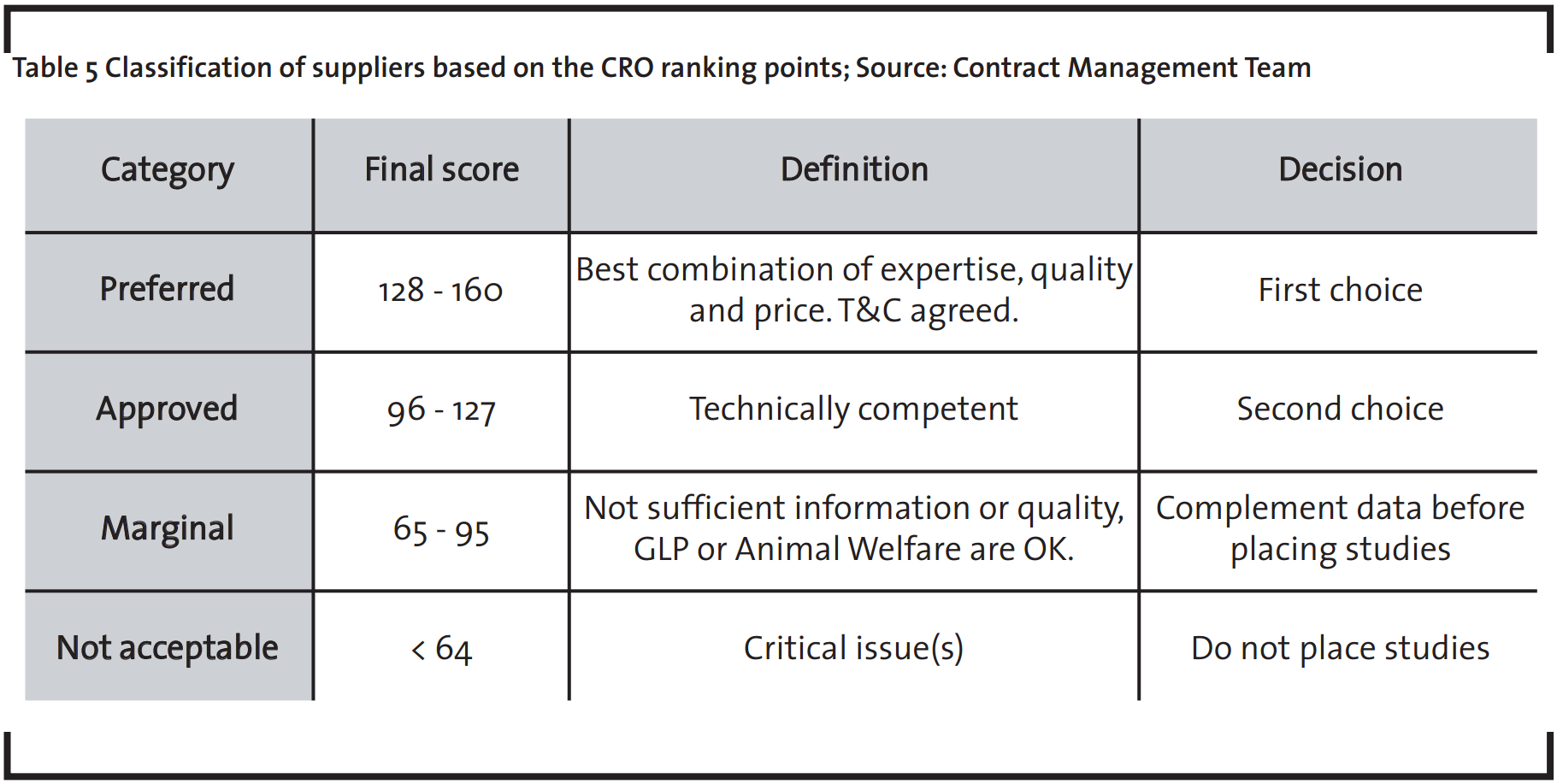
The evaluation team gives to each criterion a score from one (very bad) to five (very good), and the product of the two dimensions (weight x score) results in a total score for the CRO. The score ranking indicates which CRO is closer to the “ideal” supplier, the one that would receive the greatest possible total score.
The suppliers are classified into four categories in the Supplier Matrix: preferred, approved, marginal and not acceptable suppliers, as summarized in Table 5. Depending on the attributed importance level, the supplier must receive a score of at least 4 to be accredited as a preferred supplier (see Table 4). An example is the Animal Welfare evaluation. This evaluation system also delivers study specific information, considering differences in compliance levels and quality of one supplier.
Before the placement of any studies with a CRO, a Terms and Conditions (T&C) Agreement should be in place with the preferred and approved CROs, to avoid any potential future legal or relationship issues. It is recommendable to select at least one preferred supplier for each technical area or discipline.
3.6 Continuous evaluation of suppliers
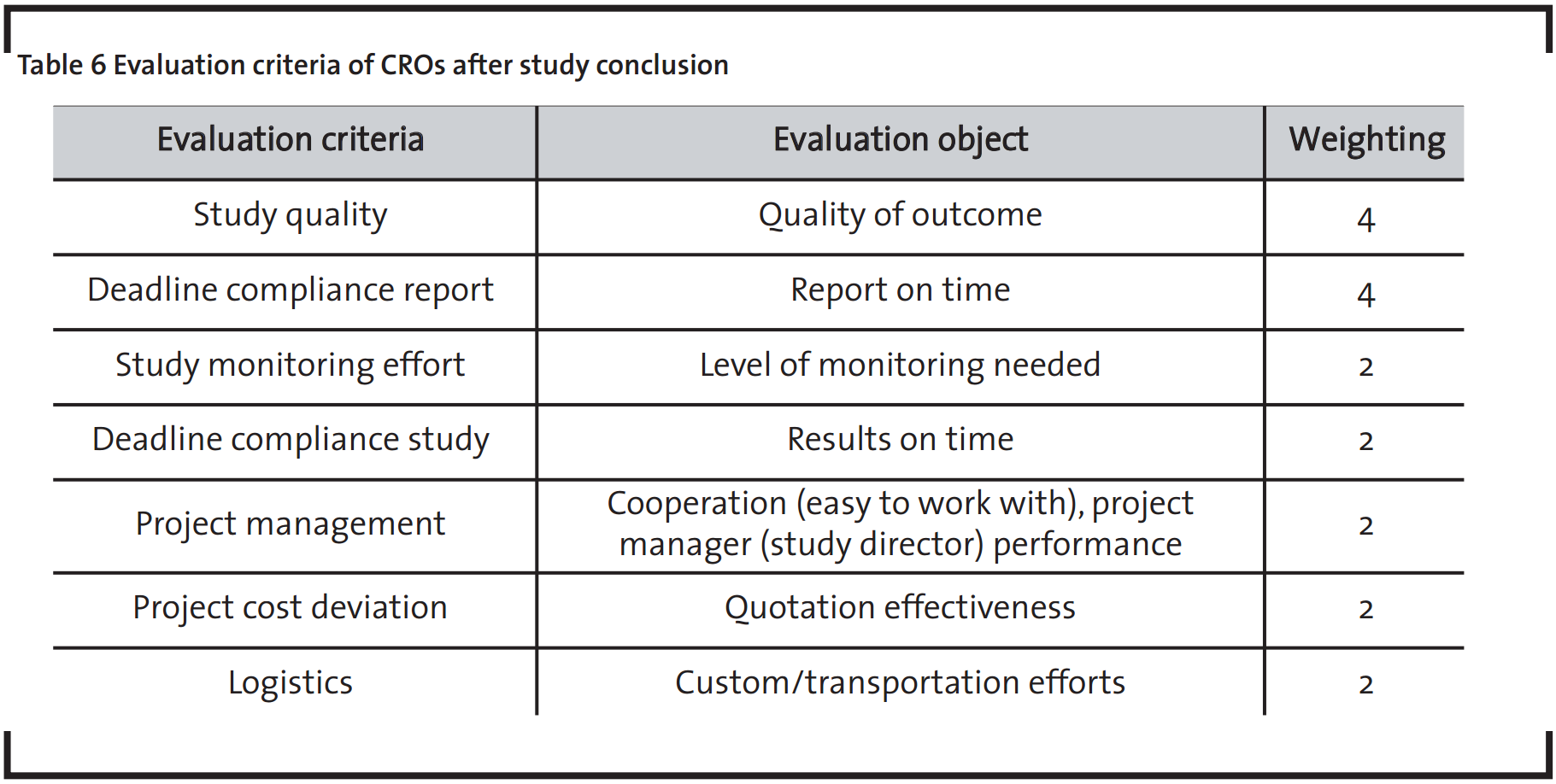
A further important step in outsourcing risk mitigation is the continuous evaluation of suppliers’ performance. Feedback should be collected after each study/project conclusion based on evaluation according to a defined set of criteria. The evaluation criteria comprises technical and non-technical aspects, focusing on study parameters like quality, delivery on time, project management and cost deviation.
The evaluation criteria and respective importance level are shown in Table 6. The study monitor provides feedback on the supplier performance by attributing a score between one and five to each criterion. The product between score and weight will generate the total score that the CRO reached for the specific study type (the same as for the selection process).
This second stage provides information for the annual Supplier Matrix update, together with other factors such as staff movements, new competitors or price changes. Another key advantage is that it generates a performance databank, enabling comparisons between different suppliers and different study types.
Regular meetings involving both the sponsor and CRO representatives should take place, depending on the volume and frequency of work placed with the supplier. This should be a forum for discussions about the issues highlighted in the evaluation and is designed to contribute to the on-going relationship development with the supplier. For major suppliers it is recommended that reviews take place more often. Supplier meetings should:
- Provide an arena for dialogue between suppliers and clients at both team and senior level
- Encourage mutual understanding of roles and responsibilities, goals and objectives
- Promote knowledge sharing based on objective metrics, surveys and lessons learned.
The benefits of such forums can be experienced by both partners. Good performance can be transferred to other study types and/or CROs. Conversely, bad performance, unrealistic requirements, outdated performance indicators, portfolio changes, current and potential pricing changes, etc., should be discussed in these meetings as the basis of corrective and preventive actions. The lifecycle can be improved through activating the controlling mechanisms, and the stage where the lifecycle re-starts depends on the action required.
3.7 Defining metrics for the outsourcing performance measurement
It is not sufficient to understand how efficient and well a supplier performs studies but also how effective the relationship is or to what extent the relationship brings added value to the sponsor.
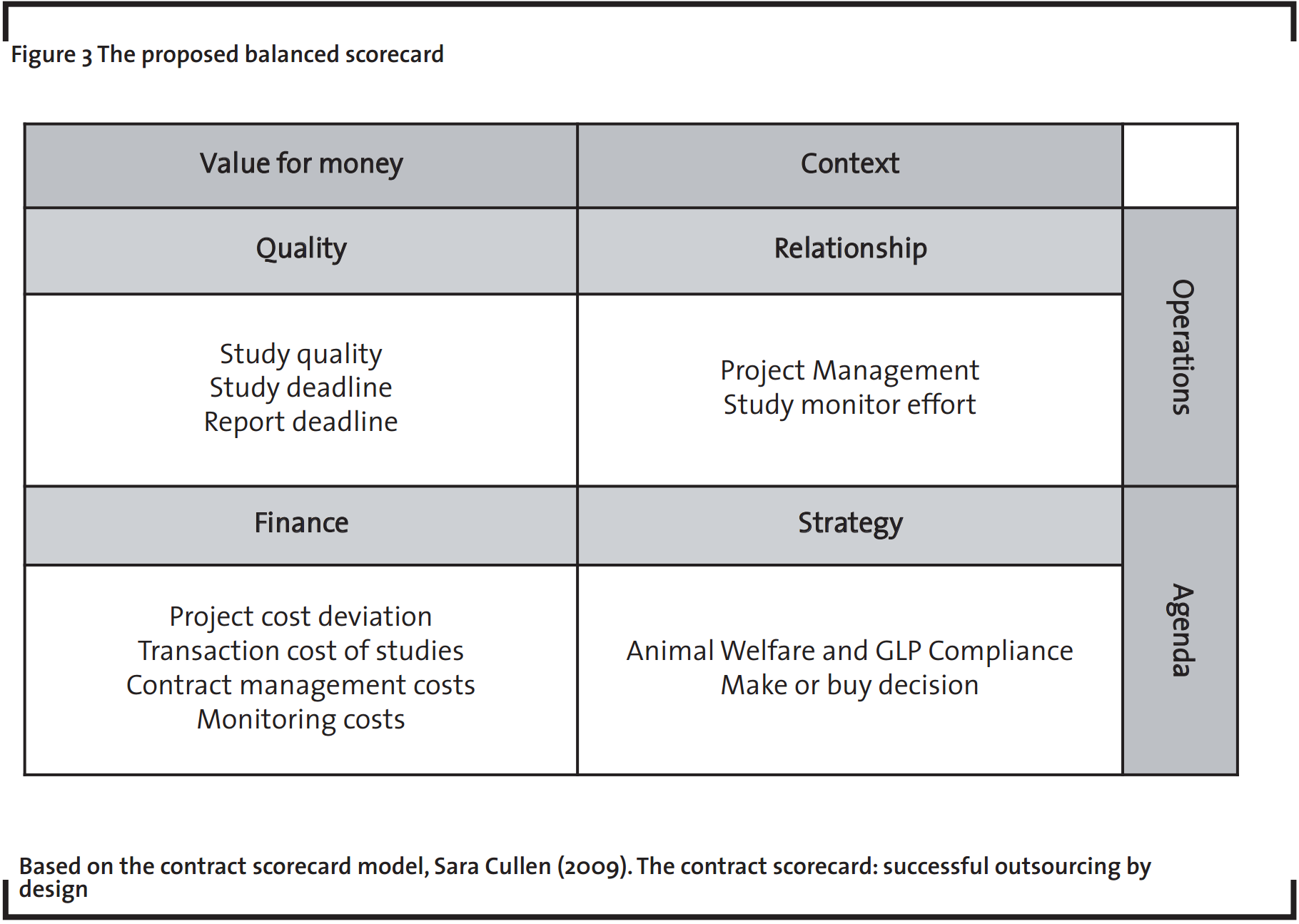
Performance measurement is a critical element to any strategy; it acts as a “reality check” between the planned strategic objectives and the results. The principle of “what is measured is managed” highlights that KPIs provide a realistic picture of what is in place and allow strategic and tactical corrections. The author Sara Cullen (2009) proposes performance evaluation through a contract scorecard – a balanced scorecard in the context of the contract management. It is composed of four quadrants: quality, finance, relationship and strategy, as illustrated in Figure 3.
Some KPIs have been defined to monitor the supplier selection and evaluation processes, e.g. study quality, study and report deadlines as well as project management evaluation. They are the essential measurement points of both output quality and the efficiency of the relationship. The study monitoring effort represents another critical factor for success of outsourcing. The scores attributed by the study monitors are the basis for a qualitative measurement. A ratio between the cost of personal dedicated to study monitoring and the number of studies outsourced is included in the finance quadrant of the scorecard because it quantifies the demanded effort level to monitor studies.
Still with regard to financial performance, the transaction cost is included in the quadrant as a metric of outsourcing efficiency. The CRO cost and the additional internal costs incurred to manage the outsourced studies should not be higher than the internal cost of performing the study in-house. If the internal and external costs are equal, it is not worthwhile outsourcing from an economic perspective.
A further financial measure that is included in the scorecard is the project cost deviation, indicating the planned and the actual amount spent. Additional KPIs to measure the outsourcing efficiency are required to monitor the cost evolution of the contract management: the increase in the contract management team costs in comparison to the increase in the total contract budget as well as a ratio between the contract-out team cost and the outsourcing volume.
With the gathered information about internal costs and the suppliers’ quotation the margin generated in each outsourcing transaction can be calculated. This reveals a potential cost reduction in some outsourced study types. However, other studies have to be outsourced due to a lack of internal capacity. In these cases, cost reduction is not the major driver and the outsourcing may even have an acceptable negative cost impact. The internal additional management and monitoring effort are not compensated by the small margin. In some cases, it is necessary to absorb extra costs to gain extra capacity and ensure the required study delivery for the internal clients.
4 Evaluating trade-offs
The additional monitoring costs are one of the trade-offs to be balanced when outsourcing toxicological studies. The costs estimated for studies required by REACH do not take into consideration the additional internal resources required for work placement and monitoring, which involves additional personal in the contract management team and the capacity increase through external consultants. How will the current monitoring costs develop with the new structure?
Increasing internal personal levels at a higher rate than the increased volume demand for work may demonstrate that contract management costs can be higher than predicted. However, the workflow centralization could decrease costs in other areas, e.g. product stewardship and business units, or could free up capacity in these areas for other tasks: a sort of cost transfer. An alternative strategy could be the increase of internal study capacity. However, physical space for laboratory expansion would involve significant financial investment and the payoff in the long run may not be high enough. The buildings cannot accommodate more equipment or personal, which means that an expansion would be much more costly than only hiring new technicians. It would require significant investment in infrastructure, involving not only the construction of a new building, but also additional infrastructure for animal feed storage, pathology, etc. Besides, another factor is the time required.
5 Conclusion
State-of-the-art literature and industry best practices have helped to develop further the strategy and some factors can be identified as critical for its success. The entire outsourcing lifecycle has experienced a much higher level of activity, which demands closer monitoring and coordination in order to keep it aligned with the strategic objectives. The changes also rely on a more centralized workflow that requires extra resources to be put into place, for example additional capacity to monitor studies.
The establishment of outsourcing guidelines in the field of toxicology impacts not only local teams but also regional businesses. As the local processes have already been defined and implemented, they have to be adapted to the regional conditions and structures, so that, in addition to the existing guidelines on compliance (e.g. Animal Welfare), the organizational standards can be globally followed. Strategy coordination and study placement monitoring worldwide generates an additional workload.
An important information source was the questionnaire, developed during this work to obtain information about the CROs regarding the most important aspects for selection and evaluation. Combined with the Animal Welfare questionnaire, it acts as a basis for decisionmaking when selecting new suppliers, delivering information to the organization about the supplier’s capabilities and strengths. The supplier matrix represents a further potentially sensitive point for internal staff, because it depends on the understanding and adoption by the contract management team and study monitors. To ensure the system’s effectiveness, the people that interact with CROs must feed it with accurate information and update it regularly. The evaluation system is essential for supplier management, because it provides information about their performance and enables corrective actions.
The supplier matrix and performance evaluation concepts are dynamic systems. As the process evolves, KPIs can become irrelevant while others can be augmented to help the organization improve its efficiency and therefore should not represent an extra workload but have a direct benefit. A next step would be to define the desired standards for each metric and follow them periodically.
Appendix
Appendix I – BASF SE animal welfare policy (extract thereof) B
- ASF´s animal testing is conducted according to legal requirements and [in Germany] in compliance with the German Animal Welfare Act, and the relevant EU directives. Furthermore, BASF is committed to high ethical and scientific animal welfare standards. Its experimental toxicology and ecology unit is accredited by AAALAC (Association for the Assessment and Accreditation of Laboratory Animal Care). Most of the practical work with animals is carried out by BASF’s Experimental Toxicology and Ecology.
- In those cases where BASF needs to commission animal studies to external contract research organisations, scientific and animal welfare monitoring is performed by experts of BASF’s Product Safety to ensure that our internal ethical and animal welfare standards are maintained on a global level.
Appendix II – Suppliers’ questionnaire
Supplier’s Name:
Contact person:
Function:
1. Human resources
We would like to gain an overview of the human resources in your company, i.e. number of employees dedicated to the different fields. Please specify number of scientists and technical staff.
- What educational background do the Study Directors have?
- How are the technical experts trained?
2. Expertise and experience
We would like to gain further information about the technical expertise and experience of your company.
- How long have you been performing studies?
Toxicological ______ years
Ecotoxicological ______ years - How many studies (per type) are performed a year?
- Please indicate the 3 main areas in which your company specializes.
- In which areas do you consider your company to be most competitive?
3. Economics
We would like to gain an insight into the size and financial performance of your company.
- Please indicate in percent the revenue distribution of your company for the industries and regions quoted.
- How has your company developed financially over the last 5 years?
4. Technology development
We would like to gain an overview of investment plans on pre-clinical testing for the next years.
5.Market information
- Please estimate your percentage of market share in the regions.
- Please indicate in percent your expectation for development over the next five years.
- Is REACH impacting your business? How?
6. Additional comments
This section may be utilized for any additional comments and/or site-specific information regarding your company.
References
Bozarth, C. C., Handfield, R. B. (2006): Introduction to Operations and Supply Chain Management, 2nd ed., Pearson International Upper Saddle River, New Jersery.
Cavusgil, Knight and Riesenberger (2008): International Business: Strategy, Management and the New Realities, Pearson International Edition, Upper Saddle River, New Jersery.
Cullen, S. (2009): The contract scorecard: successful outsourcing by design, Gower Publishing Limited, Surrey, UK.
Festel, G., Schicker, A., Boutellier, R. (2010): Performance improvement in pharmaceutical R&D through new outsourcing models, Journal of Business Chemistry, 7(2), p. 89-96.
Fleischer, M. (2007): Testing Costs and Testing Capacity According to the REACH requirements: results of a survey of Independent and Corporate GLP Laboratories in the EU and Switzerland, Journal of Business Chemistry, 4(3), p. 96-114
Grönroos, C. (1990): Service Management and Marketing, a CRM approach, Lexington Books, Lexington, MA.
Kerner, J. C. (2009): Erfolgsfaktoren des Internationalen Outsourcing-Projektmanagements, Kovac, Hamburg.
Mintzberg, H.; Quinn, J. B. (1996): The strategic process: concepts, contexts, cases, 3rd ed., Prentice Hall International, Upper Saddle River, New Jersey.
Norman, R. (2000): Service Management: Strategy and Leadership in Service Business, 3rd ed., John Wiley & Sons, Hoboken, New Jersey.
Oshri, I., Kotlarsky, J., Willcocks, L. P. (2009): The Handbook of Global Outsourcing and Off-shoring, Palgrave Macmillan, London.
Peukert, J., and Sahr, K. (2010): Sustainability in the chemical and pharmaceutical industry – results of a benchmark analysis, Journal of Business Chemistry, 7(29); p. 97-106.
Plankenhorn, S. (2008): Innovation Off-shoring: from cost to grow; analysis of innovation off-shoring strategy with evidence from European sponsors and Asian contract researchers, Gabler, Wiesbaden.
Power, M. J., Desouza, K. C., Bonifazi, C. (2008): The Outsourcing Handbook: how to implement a successful outsourcing process, Kogan Page, London.
Weiner, G. (2009): Service Reporting in Outsourcing Controlling, Gabler, Wiesbaden.
Wheelen, T. L., Hunger, D. L. (2008): Strategic Management and Business Policy: Concepts and Cases, Pearson Education (US), Upper Saddle River, New Jersey.
Winter, J., Baguley J. (2006): Outsourcing Clinical Development: strategies for working with CROs and other partners, Pharmaceutical Contract Management Group (PCMG), Gower Publishing, Hamshire, England.