Improving R&D productivity requires a balanced approach
In recent years pharmaceutical companies have implemented Operational Excellence (OpEx) in their R&D organizations to improve productivity but had only limited success. Practitioners and management start to question the effectiveness of process- focused improvement methodologies. In this article, we illustrate a number of challenges OpEx practitioners face and argue that our long-held assumptions and lack of understanding of pharmaceutical R&D can prevent us from seeing the real problems. We recommend a balanced approach by integrating process management with organization’s project management capability in order to better engage the stakeholders and deliver both short-term results and long-term improvement.
1 Introduction
A typical novel drug takes over 10 years and over $1 Billion from discovery to market (PhRMA, 2013). The current pharmaceutical business model is being questioned due to declining R&D productivity as measured by approved drugs over R&D investments (Cockburn, 2007; Scannell et al., 2012). One key metric of productivity is cycle time of the R&D process. Reducing the time to market has numerous business benefits, such as longer exclusivity and reduced costs (Mestre-Ferrandiz et al., 2012).
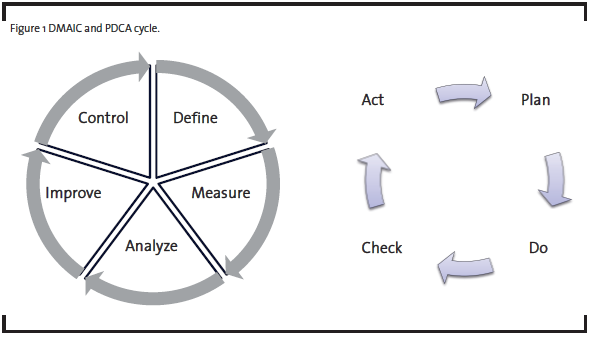
Operational Excellence (OpEx) methodologies have long been used for productivity improvement (Bertels, 2003). In the past few decades, a number of new approaches emerged. The Six Sigma methodology, introduced by Motorola in 1980’s, emphasizes the use of statistical tools to understand and reduce variation and improve quality. The Lean methodology, popularized by the success of the Toyota Production System, is widely used to eliminate waste and reduce cycle time for manufacturing and product development processes. Many organizations have combined these new methodologies with traditional quality improvement tools in their implementation of Operational Excellence. One common form of implementation is DMAIC, which is the acronym for Define, Measure, Analyze, Improve, and Control, a five-phase process improvement approach originated from General Electric. As illustrated in Figure 1, DMAIC is one form of the Plan-Do-Check-Act (PDCA) cycle advocated by W. Edwards Deming for scientific problem-solving and continuous improvement (Deming, 1986).
Since the 1990’s, Operational Excellence methodologies have also been implemented to a varying degree in pharmaceutical manufacturing to reduce cycle time, improve quality, and control costs. In the past few years, as improving R&D productivity became an imperative, Operational Excellence was introduced to many pharmaceutical R&D organizations, ranging from DMAIC training of a small group of individuals to divisional deployment of a suite of OpEx methodologies. Management and OpEx practitioners believed that the same disciplined improvement approach applies to the pharmaceutical R&D process (from discovery of a drug target to application for regulatory approval) as well as manufacturing.
While the OpEx methodologies worked well in a project within one or two functional areas, practitioners quickly realized they faced new challenges when applying them to improve the core process (Johnstone et al., 2011; Barnhart, 2013). For example, engaging a local team in a Kaizen event to improve their own process is effective. But when the outcome is less defined and the processes intertwined among many organizations and geographical locations, a myriad of issues, from defining the right metric to gaining the right sponsorship, emerge. As a result, many OpEx efforts have made limited impact in R&D. Some practitioners were quick to blame OpEx maturity and organizational culture as the causes. But they are not unique to pharmaceutical R&D and cannot explain all the challenges. To understand these challenges, we must first examine long-held beliefs and practices taken for granted in manufacturing and service environments, and ask:
- What is fundamentally different when striving for productivity improvement in R&D as compared to manufacturing or services?
- What aspects of Operational Excellence are valid and relevant for pharmaceutical R&D? What is missing in the traditional approach?
The intent of this article is not to cover the full spectrum of the challenges facing OpEx practitioners and R&D business leaders. Instead, it focuses on one key question:What is the role of process improvement in transforming pharmaceutical R&D? In this article, to help readers appreciate the specific challenges, we first present a hypothetical case that is based on our real-life observations of implementing OpEx in many pharmaceutical R&D organizations. It is used to illustrate one aspect of R&D productivity improvement: cycle time reduction. We then link the challenges to the critical differences between pharmaceutical R&D and manufacturing or services. We believe that the understanding of these differences is fundamental to developing new perspectives and applying the right methodologies in pharmaceutical R&D. Finally, by considering the organizational reality in pharmaceutical R&D and applying the above understanding, we propose a practical approach to reduce cycle time and develop organization’s capability of continuous improvement.
2 The Challenge: A Cycle Time Reduction Example
To illustrate the challenge in applying the traditional process improvement methodologies in pharmaceutical R&D, consider the following scenario.
In a global pharmaceutical company, a number of R&D programs were facing competition from multiple industry leaders, in some cases several months to a year behind the leading competitor. Based on external benchmarking, the executives estimated that their company’s overall R&D cycle time was about the same as or slightly longer than the competitors. Therefore, it was imperative to reduce the cycle times in the process wherever possible. A cycle time reduction project was chartered to identify opportunities to streamline a sub-process that had the worst performance. The team was led by a respected functional leader and coached by an Operational Excellence veteran experienced in improving both manufacturing and transactional processes in other industries, and followed the popular DMAIC framework.
As a high-priority project, the team received strong sponsorship from top management and dedicated resources, both of which were uncommon in the early stage of OpEx deployment. As a result, the Define phase went smoothly, and the sponsors approved the project with a goal of reducing the average cycle time of a sub-process in Phase II clinical development by 3 months.
As soon as the team entered the Measure phase, however, they encountered major obstacles:
- The process was poorly defined and documented in various formats. The process flow varied for each program because teams developed their own way of performing common tasks. No standardization was attempted.
- Each program required some unique decision points, branches, loops, and steps in the process. No single process map could adequately describe the current state.
- With regards to cycle time, there was no operational definition or systematic measurement. The data available was incomplete and inconsistent.
The process was obviously not consistent, outliers common, and the measurement system not reliable. If the baseline was undefined, what should be the SMART (Specific, Measureable, Achievable, Relevant, and Time-bound) goal statement at the end of Measure? Should the project goal be 1) reducing the variation to have a better defined baseline or 2) reducing the average cycle time as originally chartered? Could the team achieve both objectives?
In Analyze, the cross-functional team worked to identify the root causes of the long cycle time and variation. Some common potential causes were identified based on limited information of previous programs, including
- Resource constraints (both internal and external)
- Technology/IT limitations (multiple conflicting legacy systems)
- Regulatory requirements (unclear or changing)
- Product-specific requirements (translated into unique tasks and deliverables)
- Program priority (due to commercial/business reasons)
- Unexpected internal/external events (leading to change in resources and course of action)
Most of such factors were categorical and attribute variables that were rarely operationally defined and hard to quantify. Given the poor data available for the few dozen programs, statistical analyses typically required in Analyze were not possible. For each program, a different set of factors seemed to contribute to cycle time. There was hardly any Pareto effect or consensus on the critical few drivers. What could the team do to verify the relative impact of the factors and prioritize them for improvement?
Entering the Improve phase without a clear focus on a few critical factors, the team brainstormed and developed an assortment of solutions to address various causes. Eliminating various types of waste led to a number of proposed changes. Implementing automation to replace manual work was a popular high-impact solution but required capital investment and long-term planning. A final recommendation was to identify best practices and incorporate them into a global process as the future state. Process maps, responsibility matrices, guidance documents and templates were proposed to guide the functions involved. During the implementation, a practical question then arose: to what extent should we standardize or prescribe the solution without being seen as over-controlling or bureaucratic? In an organization where the term “process” was often viewed as a rigid structure that stifled innovation and individual creativity, how can the process be efficient, consistent, but also flexible and enabling? The team knew that ownership and change management would be a big issue in the Control phase.
The improvement project did not end with implementation, even with sustained ownership of the solutions. Despite visible process changes, new roles and responsibilities, training of staff, and a wide array of tools and templates, senior management’s question remained: “How can we be sure that the new process is better, and by how much? Can you assure us we will beat the competition?” Given the numerous changes happening outside the project and many factors (from known and controllable to unknown and uncontrollable) that could influence the cycle time, the team saw no single factor that could be a useful leading indicator. Since it would take at least two years to have one project go through the new process and many more years to establish a new baseline, the team was struggling to provide a convincing answer. In the meantime, R&D program managers asked: “How much could the new process improve MY program’s cycle time?” Improvement in the average did not matter to them because each program was so unique. Without a clear path forward, senior leadership reluctantly declared project success so they could dissolve the team to free up resources, but promised to “study the issue further.”
As the example shows, even a well prepared OpEx team with strong leadership support faces serious challenges when it comes to tackling process improvements in an R&D environment. In reality, the situation is often worse because most R&D organizations exhibit a low level of OpEx maturity: poorly defined problems, lack of sponsorship, project variability, and inexperienced teams. But what is really unique about improving pharmaceutical R&D processes? How should OpEx professionals approach it?
3 Challenging assumptions: Pharmaceutical R&D is different
To create a right mindset when approaching the R&D process improvement challenge, it is helpful to revisit two unquestioned assumptions we carry over from improving manufacturing or services.
Assumption 1: Variation is always undesirable. This is not the case in R&D. The output of the pharmaceutical R&D process is a new medicine, whose ideal characteristics are often defined by the Target Product Profile. The path to achieve that goal is much less defined at the beginning. The process involves numerous decisions along the way, e.g. choices among multiple biological pathways, mechanisms, or formulations. The decision to investigate or pursue one way or the other depends on the experience and human judgment of the knowledge workers. Some observed variability simply reflects the freedom given to the teams to design their own work and to innovate. Additional variation among projects is due to scientific or business reasons. For example, the therapeutic area influences the design and duration of a clinical investigation, and portfolio priority leads to varying levels of resource allocation among projects. The challenge is to identify and separate sources of intentional variation from those undesirable, such as irreproducible results and inconsistent project planning.
Assumption 2: The process, not the people, contributes predominately to the quality and performance. Because the work is defined and executed by a team of experts in R&D, “people are the process.” While a large amount of work in R&D is routine and can be organized and managed as a process, significant progress and innovation come from people working together as a self-organizing team. People familiar with team dynamics understand the challenge of working with cross-functional, multi-cultural, and multi-regional teams. Team leaders and senior management need to understand the factors that build an effective team, e.g. commitment to goals, clear roles and responsibilities, established trust and agreed collaborative processes. In addition, multiple teams are involved along the process from early discovery to commercialization, and ownership continuity and knowledge transfer are largely facilitated by people. Selecting the right teams and enabling them to effectively bring out innovation is not a process issue – it requires proper organization design, effective governance, a supportive culture, individual and team accountability, and continued development of people. An overemphasis on process can limit our ability to see broader issues and opportunities that related to the organization, people and culture, and therefore not engaging the right stakeholders, e.g. senior leadership.
In the hypothetical example above, the team failed to understand the sources of the variability in R&D, and approached the problem as if all observed variation was the characteristics of the process, i.e. “common cause” variation. In reality, the largest contributor is “special cause” variation, e.g. decisions and circumstances that are unique to individual programs and teams. Furthermore, the team treated the process as a manufacturing or transactional process and failed to recognize there was a large governance component in the long cycle time.
If a traditional process-focused improvement effort was not enough, how could they have done it differently?
First, there are a few concepts that are fundamental to our understanding of pharmaceutical R&D productivity.
- R&D is largely knowledge work in which a considerable number of tasks cannot be well defined. Peter Drucker raised “knowledge-worker productivity” as a management challenge in the 21st century and laid out the six major factors that differentiate knowledge-worker from manual-worker productivity (Drucker, 1999). Traditional process-focused improvement methods were developed primarily for manual work and have a basic assumption: most if not all tasks can be pre-defined in a process in order to produce the desired outcome. Consequently, we expect the process to vary within a definable range. In other words, it should be stable. To improve manual-worker productivity, the question is about “how.” This is true in manufacturing or services. However, in knowledge work, such as in R&D, the crucial question is about “what” because all tasks are not known in advance. A substantial amount of tasks are progressively planned, defined and executed by the knowledge workers themselves (individually or in collaboration). It is important to appreciate the emergent nature of R&D, in which new scientific and market information continues to emerge and modify the subsequent activities.
- A process has to be purposely designed – “fit for use.” A process in R&D should enable knowledge work productivity, e.g. enabling close collaboration and effective communication. The resistance against “processes” in R&D often comes from the perception that a process limits intellectual freedom. When designing or changing a process in R&D, we have to strike a careful balance between implementing best practices and encouraging creativity. Choosing the right processes to improve and designing to the right level of details become critical.
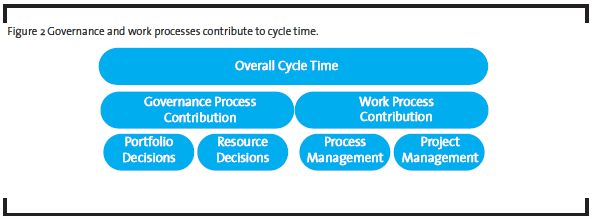
- Governance and work processes jointly contribute to R&D productivity. Governance processes determine what is done, and work processes describe how things are done. A governance process helps management establish priorities and make critical decisions regarding project portfolio and resources allocation in order to achieve optimal results. A work process represents how resources (human and non-human) are organized, based on the current knowledge and practices, to produce a pre-specified outcome. While the work processes are typical targets for improvement by OpEx practitioners, the governance processes are often the bottlenecks in R&D productivity. A different approach is required to address governance processes.
Back to our example, the team could separate the governance layer of the overall process from the operational layer, and analyze them differently. Much of observed cycle time variation was likely due to portfolio and resource decisions, i.e. how many projects in the portfolio and how many resources allocated to each project, made by the executives or governance body (see Figure 2). It would be a waste of time trying to understand why low priority projects with fewer resources took longer to complete – the time-resources relationship was known when the resource decision was made. These decisions do not change the operational level detail of the work, i.e. the work process. To reduce the overall cycle time, the management could follow Little’s law, which states that the average cycle time is proportional to the number of items in the process (“work-in-progress” or WIP) and inversely proportional to the throughput. In the case of pharmaceutical product development process, any project, whether it is active, pending, on-hold or even discontinued, is a WIP item as long as it consumes organizational resources. It is common that projects in the portfolio stop moving while the teams wait for management decisions on resources or directions. Therefore, the obvious improvement opportunities at the governance level include 1) reduce the number of projects in the portfolio and 2) simplify decision making along the critical path to allow continuous flow. The former requires disciplined portfolio management, and the latter requires the management to see themselves as servant leaders, not as authorities.
Second, the team could use the approach for managing special cause variation, i.e. identify and understand outliers. At the core of Operational Excellence is identification and separation of sources of variation. This is true and applicable in pharmaceutical R&D. But the sources in pharmaceutical R&D are much less obvious than in manufacturing or services. Instead of looking for a “process” baseline (i.e. average cycle time of the process), it would be more productive to investigate if a “project” baseline (i.e. the initial project plan) was created for each project. They could estimate the amount of variation among project baselines that reflected intentional or assignable variation, i.e. their portfolio priority and scientific rationale. The remaining variation could be attributed to two sources: 1) process inconsistency and 2) deviations in project execution. If of a significant size, each source would be opportunities for focused improvement with traditional OpEx tools and methods. By developing greater appreciation of the nature of knowledge-based work and the roles of governance and work processes in pharmaceutical R&D, we can start to see new opportunities beyond the process.
4 An Opportunity: Process and Project Management
Operational Excellence is more than a set of tools. It is a culture of continuous improvement that requires commitment and engagement at all levels across the organization. In manufacturing and services where processes are more defined, process management (with its roots in the PDCA cycle) has proven to be an effective mechanism to engage the right stakeholders in continuous improvement. Given the unique nature of pharmaceutical R&D, how can OpEx practitioners use process management effectively?
In pharmaceutical R&D, many activities change due to unique requirements of the projects or rapid advancement of knowledge and technology. Therefore, activities are more project- than process-driven. By Project Management Institute definition, a project is “a temporary endeavor undertaken to create a unique product, service, or result.” (PMI, 2013) Most of the pharmaceutical R&D activities fit this definition. Process management is useful only to the extent the activities are repeated, whereas project management capabilities are specifically developed to address the unique nature of a project.
Project management has evolved as a discipline in the past decade. As it becomes an essential part of product development and technology implementation in many industries, it has incorporated a wide range of management best practices, including Operational Excellence concepts and tools. Unfortunately, in most organizations inexperienced staff performs project management work. Too often, management and staff equate project management to “Gantt chart” management, i.e. creating and updating project schedules on a Gantt chart. Instead of proactively and methodically managing various aspects of a project, these project managers take orders from management, record meeting minutes, update the Gantt charts, and track down late tasks. In our view, project management goes beyond the standard scope, time, and cost management. It systematically integrates these aspects with quality, risk, communications, procurement, and stakeholder management required in a project lifecycle (PMI, 2013). The principle of progressive elaboration is particularly applicable to the emergent nature of R&D, and its emphasis on iterative planning and lessons learned is consistent with the PDCA cycle in Operational Excellence.
One advantage of strengthening project management is that its implementation immediately benefits the current business. Another advantage is its real-time monitoring of project metrics, such as deviations from the plan using earned value, which can be leading indicators of process performance. In addition, there is a growing acceptance of project management in the pharmaceutical R&D organizations as scientists as well as management recognize its benefits. Therefore, there exists a great opportunity for OpEx practitioners working in this industry to leverage this body of knowledge and organizational capability for improvement.
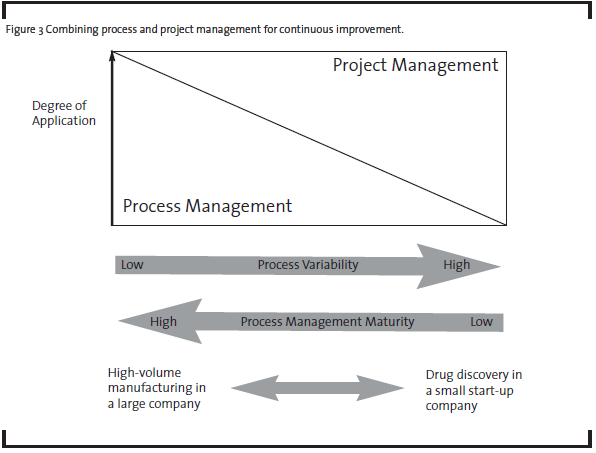
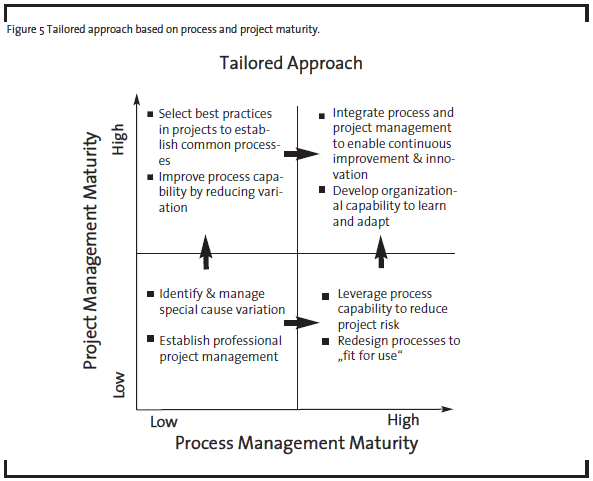
As a first step, OpEx practitioners have to see the problems from both project and process perspectives. Process management and project management can be combined to different degrees depending on the variability of the process and organization’s process management maturity (see Figure 3). For example, in a typical high-volume manufacturer where low process variability is desired, process management is most critical in the pursuit of continuous improvement. As process management is developed and matured, process variability goes down and quality and productivity go up. In the case of pharmaceutical R&D, the discovery phases have less defined processes than early and late development phases. Therefore, process management has been limited to routine activities or at a high level in discovery, but adapted more broadly in late development processes. Research and development activities in all phases heavily depend on project management. So improvement in project management capabilities will have the biggest impact on R&D productivity.
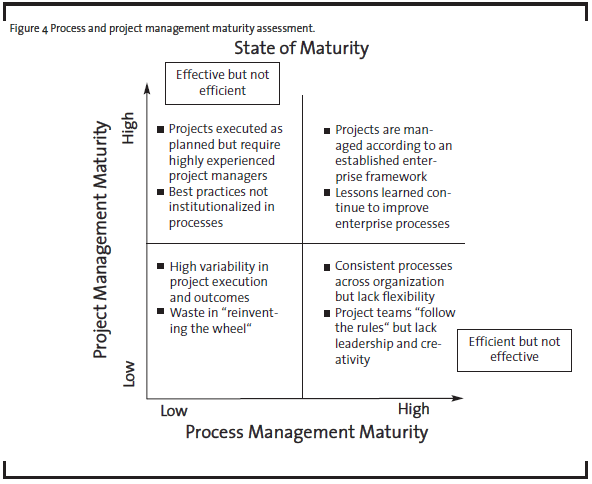
To determine the best improvement approach, it is helpful to assess the organizational maturity in two dimensions: process management and project management, as illustrated at high level in Figure 4.
For example, a R&D organization with high project management maturity but low process management maturity may be able to execute projects successfully but only with highly experienced project managers and teams. The capability is not readily scalable if the demand rises because the best practices have not been institutionalized in the form of processes. In contrast, an organization with high process maturity and low project management maturity may be efficient delivering standard or routine results. But if the external circumstances demand different outcomes, the organization may not have the right leaders and culture to respond quickly. OpEx practitioners and senior management should use this maturity assessment with their business strategy to determine the desired future state for the organization, and then identify the optimal path (see Figure 5).
Based on our experience, there is a greater organization capability in project management than process management in pharmaceutical R&D. In such a case, the organization’s project management capability should be leveraged by OpEx practitioners, i.e. project management can be a temporary substitute for process management, providing an alternative path to improved business results until the organization becomes ready to embrace full process management. The same maturity assessment can also help other R&D organizations, such as those in the medical device industry, where both process and project management have a medium level of maturity. OpEx practitioners improving R&D processes should consider the following steps.
- Assess organizational maturity in process management and project management
- Understand what is common among R&D projects and what is unique
- According to the organizational maturity, apply process management to standardize the common tasks as processes, and apply project management to manage unique tasks in projects, respectively
- Build project plans based on governance decisions, standardized work processes, and expertise of the teams
- In real time, use lessons learned during project execution as feedback to continuously improve existing processes
- Continue to improve process and project management capabilities in the organization
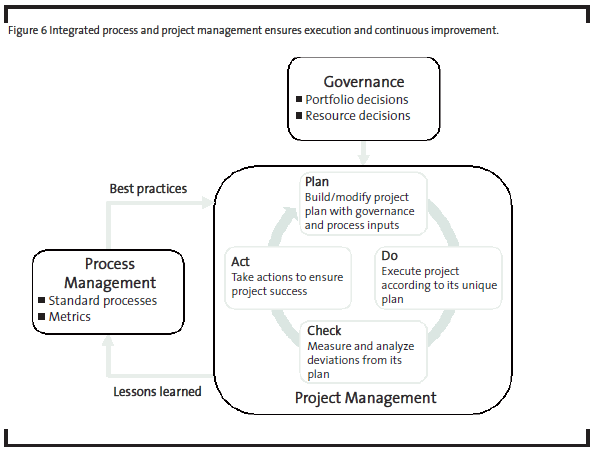
Figure 6 shows how governance, project management, and process management work together to ensure project success and continuous improvement.
5 Conclusion
In summary, improving pharmaceutical R&D using classical process-focused approaches are unlikely to succeed without adaptation to its unique environment. The emergent nature of R&D and knowledge-based work mean that R&D processes are less defined and highly variable. Governance, organizational structure and capability have significant impact on R&D productivity. Operational Excellence practitioners using the traditional approach need to look beyond the process and question long-held assumptions. One opportunity to overcome some of these challenges is to integrate project management with the Operational Excellence methods that do apply. By carefully balancing project management and process management in our approach to R&D productivity, we can continually reduce variation over the long term while improving business results from on-going R&D projects. The effectiveness of this approach will impact not only R&D productivity but also culture change required for continuous improvement by embedding the PDCA mindset in everyday activities at all levels of the organization.
Acknowledgements
We thank Fredrik S Bendiksen (former Vice-Head of R&D, Nycomed), Linda Bryant (VP, Global Strategy & Business Excellence, Janssen), Venky Gopalaswamy (Vice President and head of Business Process Management, Philips), Paul W. Knight (Director, R&D Productivity & Continuous Improvement, Bristol-Myers Squibb), Dr. Ralph N. Landau (Vice President, Development & Regulatory Affairs, Sandoz), and Joseph A. Ritter (Director, Sigma, Strategy and Operations, Merck) for their thoughtful comments and suggestions on the earlier versions of the manuscript. We also thank our colleague, Steve Crom, for critical review, feedback, and editing of the initial draft.
References
Barnhart, T.M. (2013): Creating a Lean R&D System, Taylor & Francis Group, Boca Raton, Florida.
Bertels, T. (ed.) (2003): Rath & Strong Six Sigma Leadership handbook, John Wiley & Sons, Inc. Hoboken, New Jersey.
Cockburn, I.M., (2007): Is the Pharmaceutical Industry in a Productivity Crisis? In Innovation Policy and the Economy, National Bureau of Economic Research, Lerner J. and Stern S. Ed. Volume 7, pp.1-32.
Deming, W.E. (1986): Out of the Crisis, Massachusetts Institute of Technology, Cambridge, Massachusetts.
Drucker, P.F. (1999): Management Challenges for the 21st Century, HarperCollins, New York, pp. 133-159.
Johnstone, C., Pairaudeau, G., and Pettersson, J.A. (2011): Creativity, innovation and lean sigma: a controversial combination? Drug Discovery Today 16 (1/2), pp. 50-57.
Mestre-Ferrandiz, J., Sussex, J., and Towse, A. (2012): The R&D Cost of a New Medicine, Office of Health Economics, London.
PhRMA – Pharmaceutical Research and Manufacturers of America (2013): 2013 Pharmaceutical Industry Profile, Washington.
PMI (2013): A Guide to the Project Management Body of Knowledge (PMBOK® Guide), fifth edition, Project Management Institute, Inc., Newtown Square, Pennsylvania.
Scannell, J.W., Blankley, A., Boldon, H., and Warrington, B. (2012): Diagnosing the decline in pharmaceutical R&D efficiency. Nature Reviews Drug Discovery 11, 191-200.