Patterns of collaboration along the bio-pharmaceutical innovation process
Abstract
Literature has widely acknowledged that creating a tight network of collaborations is an unavoidable strategy for innovative biotech firms. However, few contributions have focused so far on how collaborations along the bio-pharmaceutical innovation process are organised in practice. The paper attempts to cover this gap by investigating, on a large empirical base which covers the years 2000-2005, the adoption of different organisational modes of collaboration in the bio-pharmaceutical industry. A framework of analysis, identifying the relationship between organisational modes and the phases of the drug discovery and development process, has been developed and assessed in the industry. This has allowed to disclose the determinants of adoption of different organisational modes of collaboration and their relationship with the typology and size of partners involved. In this respect, the paper also contributes to the ongoing debate about Open Innovation, examining its organisational implications.
Introduction
The advent of biotechnology in the ‘80s deeply revolutionised the R&D and innovation process in the pharmaceutical industry. In contrast with the traditional “monolithic” approach centred on chemical pharmacology, biotechnology requires the capability to handle and integrate a number of scientific disciplines and technologies, e.g. genetics, immunology, biochemistry, general medicine, computer science, physics and optical science (Byerlee et al., 1999; Powell, 1998). This had two major, although strictly interrelated, effects: on the structure and the division of labour within the pharmaceutical industry, as well as on the management and organisation of the bio-pharmaceutical innovation process. On the one side, the advent of biotechnology paved the way for the birth and proliferation of new biotech companies, highly specialised on few scientific disciplines and focused on the development of a very narrow set of technologies (e.g. bioinformatics, High-Throughput-Screening), or committed to specific tasks (e.g. screening, lead optimisation) of the revolutionised innovation process, which is still undertaken in large part within the boundaries of established pharmaceutical firms (Chiaroni et al., 2008; Chiesa, 2003; Malerba and Orsennigo, 2002; Muffatto and Giardina, 2003).
On the other side, for both small biotech firms and traditional pharmaceutical companies, it became hardly impossible to effectively and efficiently manage the whole innovation process within their own boundaries, because of the high number of scientific and technological competencies to be contemporarily mastered. As a result, building a network of inter-organisational R&D collaborations that acts as a coordination means among different actors (e.g.new biotech companies, established pharmaceutical firms, but also universities, research centres, science parks), each contributing to the innovation process with its own competencies and technological assets, turned out to be a strategic imperative (Chiesa and Toletti, 2004; Niosi, 2003; Powell et al., 1996). In recent years, partially as a consequence of the globalisation of markets and the increasing diffusion of biotech applications, R&D collaborations along the bio-pharmaceutical innovation process have gained further momentum and nowadays they represent one of the key drivers of industry growth (Baum et al., 2000).
As a result, literature has widely investigated the topic from several different, although complementary perspectives (Deeds and Hill,1996; Dussauge and Garrette, 2000; Gulati, 1998). This paper contributes to this stream of research, focusing on biotech firms operating in the pharmaceutical industry by empirically analysing: (i) the extent to which biotech firms adopt collaborations in the drug discovery and development process; (ii) the organisational modes selected for these collaborations; (iii) the type of external partners involved; (iv) the evolution of the organisational modes and of the type of external partners along the different phases of the biopharmaceutical innovation process.
This paper is believed to add also to the recent debate on Open Innovation (Chesbrough, 2003; Chesbrough et al., 2006; West et al., 2006). In particular, it is one of the few contributions, to the best of our knowledge, that provides some insights on the degree to which biotech firms conform to the Open Innovation philosophy, studying their attitude to exploit collaborations with external partners, and on the different organisational modes through which the Open Innovation paradigm is implemented.
The paper is structured as follows. The next section briefly reviews the literature on R&D collaborations in the bio-pharmaceutical innovation process and the Open Innovation model, whereas the third part describes the research strategy adopted in the paper. The fourth section reports and discusses the results of the empirical analysis; finally, some conclusions and future directions of research are outlined.
Collaborations in the bio-pharmaceutical innovation process and Open Innovation: a literature review
R&D collaboration can be defined as the practice through which a firm establishes a relationship with an external organisation with the purpose of improving the performance of its R&D processes (Chiesa and Toletti, 2004). Literature has widely acknowledged that the creation of a tight network of R&D collaborations with a range of external partners is an unavoidable strategy for innovative companies in the bio-pharmaceutical industry (Barbanti et al., 1999; Fontes, 2003; McKelvey et al., 2003; Niosi, 2003; Powell et al., 1996; Salman and Salves, 2005).The development of a novel drug according to the new biotechbased R&D process requires, indeed, the convergence of many sources of knowledge and skills. Therefore, networks of collaborations turn out to be an effective means of industrial organisation along this complex R&D process.
As a result, scholars have claimed that the formation of strategic R&D collaborations is a key factor explaining the survival and growth of smaller biotech firms (Audretsch and Stephan, 2001; Niosi, 2003) focusing either on the development of supporting technologies or on specific tasks of the whole R&D process. At the same time, however, strategic R&D collaborations also explain the growth of large “traditional” pharmaceutical companies. Establishing a network of collaborations with innovative biotech firms, large pharmaceutical companies have succeeded in facing the challenges of the so-called “biotech revolution” and in keeping their dominant position in the industry. Moreover, as suggested by Powell et al. (1996),in the bio-pharmaceutical industry this network of collaborations increasingly involves partners different from biotech or pharmaceutical companies, such as universities, public research laboratories and private investors.
In recent years, there has been an unprecedented growth in strategic R&D collaborations in high technology, and especially science-based industries. This trend is particularly evident in biotechnology,as shown by practitioners and consulting companies operating in this field (Burrill&Company, 2005; Ernst&Young, 2004; Ernst&Young, 2005; Ernst&Young, 2006).
As a result, several scholars have been investigating the topic, covering a wide array of aspects. The largest part of the contributions on collaborations in the bio-pharmaceutical innovation process focuses on: (i) the impact of collaborations on the innovative performance of the focal firm (Baum et al., 2000; Deeds and Hill, 1996; Gulati, 1998; Lerner et al., 2003); (ii) the impact of collaborations (and particularly of in-licensing) on the productivity of large pharmaceutical companies (Laroia and Krishnan, 2005); (iii) the role of partners’ complementarity of assets (resources,capabilities, or knowledge competences) on the forms selected for collaborations (Helfat, 1997; Liebeskind et al., 1996); (iv) the reasons for success and failure in R&D collaborations (Dussauge and Garrette, 2000; Lane and Lubatkin, 1998).
Although this body of literature is extensive, relatively scarce attention has been paid so far to the problem of how R&D collaborations along the bio-pharmaceutical innovation process are organised in practice. In particular, literature shows that a wide spectrum of organisational modes can be adopted for R&D collaborations, ranging from mergers & acquisitions, through joint ventures, alliances and outsourcing, to licensing agreements (Chiesa, 2001). Nevertheless, no systematic attempt has been made to empirically evaluate the extent to which biotechnology firms use these alternative organisational modes, and whether some sort of specialisation along the stages of the bio-pharmaceutical innovation process is in place. Furthermore, the typology of partners with which biotechnology firms collaborate in the different phases of the innovation process has been the subject of sparse research, too, although it seems a critical determinant of a collaboration’s success (Chiesa and Manzini, 1998). These issues will be dealt with at length in this paper.
As pointed out in the previous section, this paper is believed to contribute also to the ongoing debate on Open Innovation, the new paradigm for the management of innovation which conceives the firm as an open system that purposefully and systematically leverages the resources of external organisations in order to support the generation and exploitation of its innovative capabilities (Chesbrough, 2003). Academic and managerial research on this topic has been extensive (for an up-to-date bibliography on this issue see the web site:http://www.openinnovation.net); nevertheless, an important gap can be identified that is relevant in the light of the objectives of this paper, i.e. the scarce attention dedicated to the organisational and managerial implications of this new model.
It should be noted in fact that the Open Innovation paradigm, as discussed by Chesbrough and colleagues, has a very general nature, since it basically captures the underlying logic at the roots of most innovative firms’choices in the area of technology management. However, companies that are willing to implement the Open Innovation “philosophy” need to select specific organisational modes through which they can leverage their knowledge-abundant external environment. The choice of how to organise the firm’s R&D collaborations is one of these critical implementation issues. Scholarly literature has not addressed this topic systematically and in-depth so far, besides a few attempts to discuss the most appropriate intellectual property strategies (Chesbrough, 2003) and performance metrics (Chesbrough, 2004) for supporting Open Innovation, or to study the criteria affecting the choice of the governance mode for external technology sourcing (van de Vrande et al., 2006). Moreover, anecdotic evidence is available about how most innovative and successful enterprises have been managing and organising their transition towards Open Innovation. For instance, Huston and Sakkab (2006) describe the different types of networks and the strategic planning process which are at the heart of Procter & Gamble’s Open Innovation approach, which is called “Connect & Develop”; Kirschbaum (2005) explains how the multinational life cycle and performance materials company DSM has built a teamwork and entrepreneurial culture for opening up its innovation process. Nevertheless, a structured theory of the managerial and organisational enablers of the Open Innovation paradigm has not yet been developed.
This paper will help make a step further in this direction. Studying the adoption of different organisational modes for R&D collaboration in the biotech industry, it will contribute to disentangle the issue of how firms practically implement the Open Innovation paradigm. Adopting the taxonomy suggested by Chesbrough and Crowther (2006), we will distinguish between two different types of inter-organisational relationships, according to the purpose for which they are established:(i)“inbound organisational modes” (e.g. licensing-in, acquisitions, R&D contracts and research funding, alliances), which have the purpose to access technical and scientific competences owned by external partners for improving the focal firm’s innovation performance; (ii)“outbound organisational modes”(e.g. licensing-out,spinning-out of new ventures, provision of technical and scientific services), which have the purpose to commercially exploit technological opportunities developed within the focal firm.
Finally, although the paper is primarily focused on the issue of R&D collaborations organisation, it is one of the few literature contributions (Fetterhoff and Voelkel, 2006) that provides some empirical evidence of the adoption of the Open Innovation paradigm in the bio-pharmaceutical industry. This gap in the existing literature about Open Innovation is relevant since the biotechnology, and especially the bio-pharmaceutical industry, show several characteristics that make them a fertile ground for the diffusion of Open Innovation and hence for the study of the latter’s managerial and organisational implications. In this respect, it is worth remembering its extraordinary technology intensity (DeCarolis and Deeds, 1999), the complexity of the innovation process and the heterogeneity of the competence sit requires (Powell et al.,1996), the pivotal role in the development of the industry of technology transfer mechanisms (Madhock and Osegowitsch, 2000), the intensity of relationships between biotech firms, universities and research centres (OwenSmith et al., 2002) and the birth of a venture capital market, at least in Anglo-Saxon countries, specialised in supporting biotech ventures (Powell et al., 2002).
In conclusion, this brief literature review highlights the potential relevance of the managerial and research implications of this paper, both in respect to the traditional literature about collaborations in the biotechnology industry, as well as to the recent debate on Open Innovation.
Research methodology
In order to achieve the objectives of this paper, a two-step research strategy has been adopted. The first step aims at developing a reference framework to identify the critical “inbound” and “outbound” organisational modes and their relationship with the different stages of the bio-pharmaceutical innovation process. The framework, taking into account the peculiarities of innovation activities undertaken by biotech companies, allows supporting the subsequent empirical analysis. In the second step, the framework was applied to a longitudinal empirical data set, in order to test its initial validity.
As far as the first step of the research is concerned, a panel study was organised, involving 20 people (business development managers, R&D directors, chief executive officers of biotech companies, as well as academics and consultants with a significant experience in the field) among the most representative companies of the Italian biotech industry. The full list of participants in the panel study is reported in Table 1.
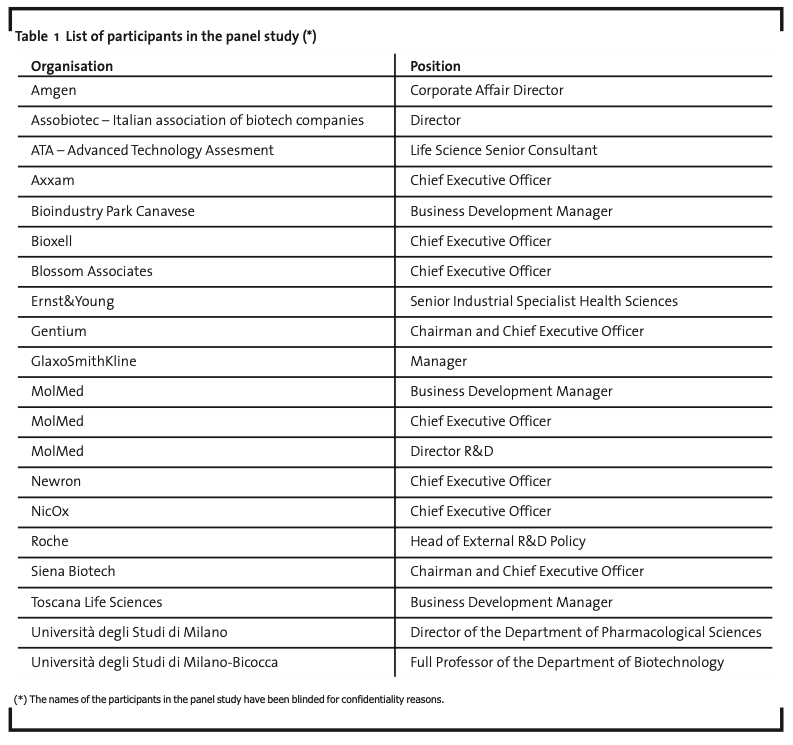
Two rounds of interviews were conducted directly by the authors. Each round allowed to accomplish a main task, respectively: (i) to share and validate a model of the actual sequence of phases that constitute the whole drug discovery and development process in the bio-pharmaceutical industry; (ii) to identify, for each of the above phases, which of the organisational modes identified by the literature are more suitable to be implemented by biotech firms. The determinants of each choice have been discussed and related to the characteristics and peculiarities of the biotech industry.
As a whole, the panel study allowed the authors to develop a framework for investigating the “inbound” and “outbound” organisational modes adopted by biotech firms for collaborating with external partners.
In the second step of the research, we selected the first 20 pharmaceutical biotech firms worldwide (considering their market capitalisation at the end of December 2006, Table 2) and, for each company, we documented the organisational modes they used in the various phases of the drug discovery and development process as well as the type of external partners they collaborated with. Some further details on the empirical investigation are provided below concerning (i) the selection of the sample, (ii) the time period covered in the analysis, (iii) the type of data collected, and (iv) the data sources.
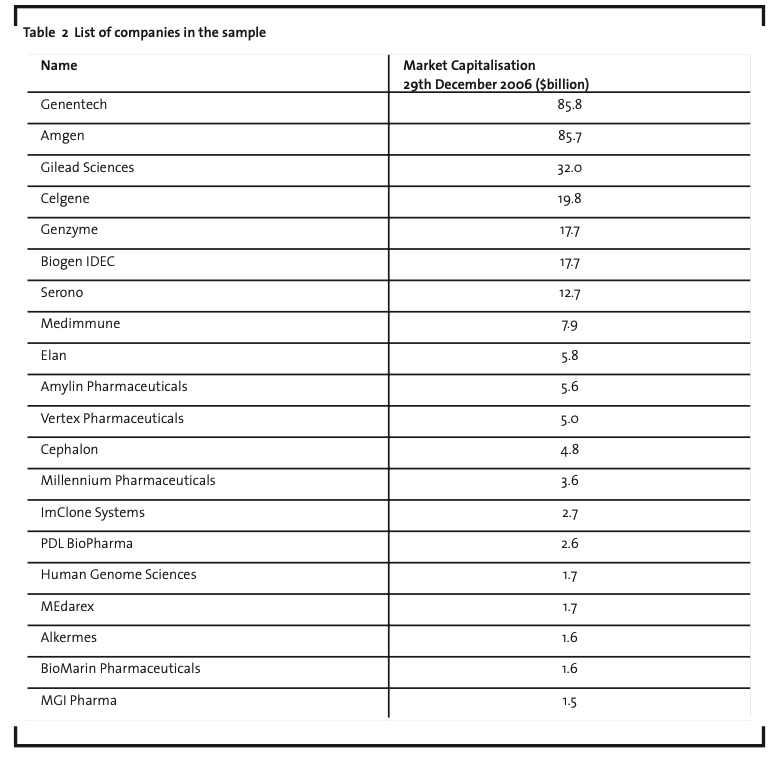
First, it is worth mentioning that the selection of the top 20 biotech firms in terms of market capitalisation is consistent with the purpose of the paper. This is true for a twofold reason: on the one side, companies listed on public stock exchange markets also have to disclose information about their R&D activities, thus allowing to access relevant information about their collaborations; on the other side, firms in the sample represent the top players in the industry and are therefore more suitable to highlight relevant trends and best practices in the management of innovation processes. The time period chosen for the analysis covers the years from 2000 to 2005, attempting to balance the relevance for the research objectives of the collected information with the efficiency of procedures for data gathering. Furthermore, it should be noted that the year 2000 represents in almost all the cases the starting point of documentations and archival records for the firms in the sample. Collected data concern:
- the number and typology of different organisational modes for collaborations (as identified in the research framework developed through the panel study) adopted by the firms;
- the phase of the drug discovery and development process to which each of the above modes refers;
- the typology of partners involved. In this case, we classified external partners along two dimensions: (i) type of organisation (i.e. pharmaceutical firms, biotech firms – further distinguished into product and platform firms, according to the well known taxonomy (Chiesa and Chiaroni, 2004), universities and research centres); (ii) size (i.e. small-medium and large firms)1;
- the therapeutic area (where applicable and following the classification proposed by the Biotechnology Industry Organisation) within which the object of the collaboration can be classified (i.e. the target disease of a new drug).
As primary source of data, the annual reports of the selected firms in the time period 2000-2005 were analysed. Nevertheless, in order to verify the gathered data, they have been triangulated with information drawn from professional databases and reports (Recombinant Capital,Biospace Directory, Canadian Biotech).
As far as the reliability of the data is concerned, it should be highlighted that, for the purpose of the paper, the identification of general trends is far more relevant than the completeness of the information for each single firm. Indeed, even if the completeness might be ensured by the fact that firms in the sample are listed on public stock exchanges, it is anyhow reasonable to expect that if there are omissions they are rather equally distributed in the sample, thus not affecting the results of the analysis. Nevertheless, it is clear to the authors that achieved results have to be further validated on a larger empirical base in order to prove their statistical relevance.
Patterns of collaboration in biotech
In this section of the paper the results of the empirical investigation are presented. Specifically, the next paragraph describes the framework of analysis developed through the panel study. In the second part of the section, the outcome of the longitudinal inquiry is discussed at length.
Patterns of collaboration in biotech: framework of analysis
During the first round of interviews, participants in the panel study were first asked to discuss the structure of the drug discovery and development process in the bio-pharmaceutical industry, as it is reported in the literature (Chiaroni et al., 2008; Chiesa, 2003; Chiesa and Chiaroni, 2004; Gassmann and Reepmeyer, 2005; Muffatto and Giardina, 2003). The purpose was to reach a consensus about the number and the content of the phases to be included in the framework for the subsequent analysis of collaborations.The structure of the process suggested by the panel of experts is reported in Figure 1.
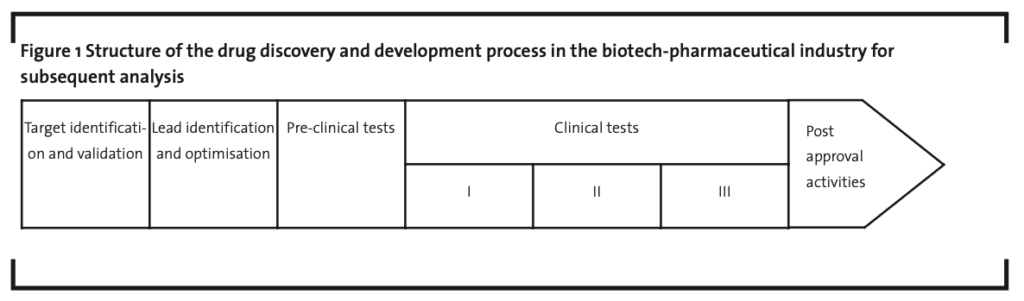
A brief description of the phases comprised by the framework follows:
- target identification and validation. Target identification has the purpose to identify a gene or a protein or a sequence of both (target), which is thought to be the pathogenic of a selected disease. Target validation, which follows immediately, concerns the study of the identified target with the purpose to: (i) define the interactions between the target and the whole human organism; (ii) check if there are intellectual property rights already claimed regarding the identified target, e.g. through accessing public databases like NCBI in the US;
- lead identification and optimisation. After assessing the genetic base of the disease’s evolution, scientists need to identify the compound that has the desired effects in treating the selected disease (lead identification).This compound actually represents the active principle of the future drug. The lead optimisation activity, finally, adds to the lead the necessary excipients (i.e.substances included in the drug formulation) in order to protect, support or enhance the stability of the active principle and to increase patients’ compliance; pre-clinical tests. This activity studies, especially through in vivo testing, the mechanisms of absorption, distribution, metabolism, excretion and toxicology of the new drug, with the purpose to evaluate its effects on animals. Before entering clinical trials, a first approval by public authorities is required; clinical tests. These trials directly involve human patients and are usually divided into three steps: phase I, phase II and phase III. In phase I, researchers test the new drug in a small group of healthy people (20-80) to evaluate its safety and to determine a safe dosage range. In phase II, the new drug is tested on a larger group of people (100-300) affected by the target disease to evaluate its effectiveness in patients and to determine the common short-term side effects and risks. Finally, the phase III involves an even larger group of patients (1,000-3,000) to confirm the effectiveness of the new drug and to evaluate its overall benefit-risk relationship. If all the three phases are successful, public authorities have to approve the new drug to allow it to be marketed;
- post-approval activities. These comprise the purchasing, production, logistics, marketing & sales and post-marketing tests for the new drug. In particular, post-marketing tests involve the monitoring of the drug’s performance along its whole life-cycle, with the purpose to delineate additional information on its risks, benefits and optimal use in the middle-term.
In the second round of expert interviews, the “inbound”and“outbound”organisational modes of collaborations were discussed, with the purpose to spot which specific modes are used by pharmaceutical biotech firms along the different phases of the development process. The interviewed managers recognised that “inbound” organisational modes take place mainly in the preclinical phase of the drug discovery and development process, i.e. target identification and optimisation, lead identification and validation and pre-clinical tests. In other words, it is chiefly on these stages that biotech companies get into contact with external organisations for leveraging their innovation efforts and accessing their highly specialised knowledge and competences. Instead, “outbound” organisational modes take place mainly in the second part of the process, i.e. in the clinical tests and post-approval activities. It is in these stages, in other words, that biotech firms generally collaborate with external organisations for commercially exploiting the results of their own innovation activities. This suggests the possibility to distinguish between two distinct macro-phases in the pharmaceutical biotech drug discovery and development process, called“generation”of innovation – where inbound organisational modes of collaboration prevail and “exploitation” of innovation – where outbound organisational modes are mainly present (Figure 2).
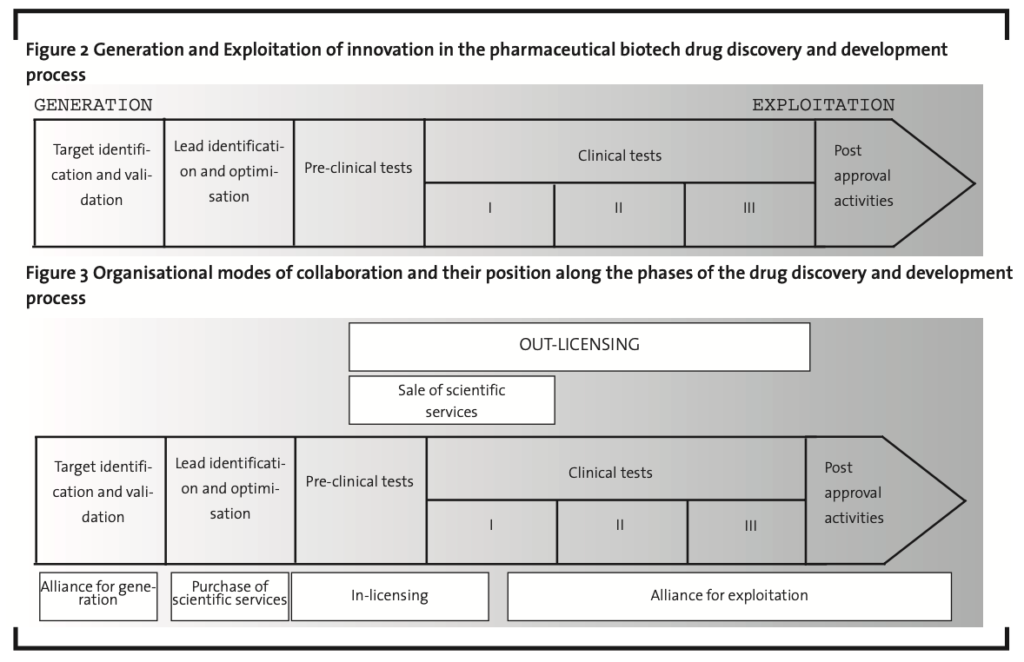
The separating point between the generation and the exploitation macro-phases was identified at the transition from pre-clinical to clinical tests. Because of the intrinsic characteristics of the biotech innovation process, in fact, it is only at the end of pre-clinical tests that drug candidates acquire the properties that allow them to be commercially exploited. Before this point, the drug discovery and development process is mainly a “trial-and-error”, internal effort characterised by extreme uncertainty and unpredictable outcomes. Once the first approval by the public authorities is obtained, at the end of pre-clinical tests, development risk is lower: the process becomes much more formalised and externally visible. At this point, therefore, actual possibilities for external commercial exploitation can be identified and pursued. Nevertheless, interviewed managers recognised a certain degree of overlapping between the generation and exploitation phases (Figure 2). This is due to the fact that, according to the characteristics of the drug under development: (i) commercial exploitation sometimes can start earlier than the end of pre-clinical tests (e.g. out-licensing of a candidate that has not completed these trials yet); (ii) the leverage on the innovative efforts of other organisations can continue beyond this limit (e.g. in-licensing of a candidate that has already completed phase I of clinical tests).
Finally, the interviews allowed identifying the specific organisational modes that pharmaceutical biotech firms use to collaborate along the phases of the drug discovery and development process:
Organisational modes of collaborations for the generation of innovation:
- alliance for the generation of innovation. In this case the biotech firm establishes a partnership (without equity involvement) with other biotech firms, pharmaceutical companies, universities or other research centres,in order to pursue a common innovative objective (e.g. the validation of a genetic target);
- purchase of scientific services. The biotech firm externalises a specific phase of its innovation process to a specialised provider (e.g. the lead optimisation activity), under a well-defined contractual agreement (for further details on the role of technical and scientific services in the biotech industry see Chiaroni et al., 2008);
- in-licensing. The biotech firm acquires the right to use a specific drug candidate from another biotech firm, a pharmaceutical company or a university.
Organisational modes of collaboration for the exploitation of innovation:
- alliance for the exploitation of innovation. In this case the biotech firm partners with another company (a biotech firm or, more often, a big pharma) for accessing some complementary assets (e.g. production capacity or distribution channels) required to commercially exploit the new drug;
- supply of scientific services. The biotech firm provides third parties (typically, other biotech firms) with technical and scientific services that leverage the outcome of its discovery efforts;
- out-licensing. The biotech firm licenses out, usually to other biotech or pharmaceutical companies, the right to use a new drug candidate that it has discovered and developed.
Figure 3, according to the results of the panel study, schematically describes the specific phases of the pharmaceutical-biotech drug discovery and development process in which these organisational modes of collaborations prevail. The next section reports and discusses the results of the longitudinal analysis, that was undertaken applying this framework.
Patterns of collaboration in biotech: evidence from the empirical analysis
The analysis of the data of the top 20 pharmaceutical biotech firms leads to interesting results concerning the patterns of collaboration in the bio-pharmaceutical industry.
First of all, it is possible to highlight a general trend (as reported in Table 3) analysing the evolution of the number of times in which organisational modes of collaboration have been adopted by firms in the sample.
The number of items recorded in the sample, 794 in total with an average for each firm of nearly 40, is significant and demonstrates, supporting the results of the extant literature on the field, the relevance of collaborations as a mean for biotech companies to sustain their business. Moreover, if the number of collaborations is assumed as a rough measure of the openness of the innovation process (as it indeed represents the number of actors involved in the biotech firm’s innovation network), the above mentioned empirical
results support also the hypothesis that the biotechnology industry (and in particular the biopharmaceutical industry) is a fertile ground for the adoption of the Open Innovation paradigm. However, it should be noticed that the trend declines from 168 items in 2000 to 113 items in 2005. As far as the determinants of this trend are concerned, at least the following two can be highlighted: (i) the impact of the overall economic context, with the blow up of the Internet (and high tech) bubble in the year 2000 and the economic downturn following terrorist attacks in 2001, reducing the availability of financial resources for biotech firms; (ii) the progressive evolution towards the maturity stage of some basic technologies (e.g. gene mapping and analysis, production of monoclonal antibodies). The former point implies an overall reduction of the innovative effort (and therefore of the number of collaborations) by biotech firms that are constrained by limited resources. The latter point implies, on the one side, the increasing concentration of the supply with a lower number of organisations offering those technologies and, on the other side, a push for larger product biotech firms towards internalising mature technologies into their own boundaries. In both cases this results in a reduction of the number of times in which firms look at external organisations to complement their internal assets and competences.
A further step of the analysis concerns the evolution of the organisational modes of collaboration in the two identified macro-phases of generation and exploitation of innovation. Table 3 shows the clear prevalence of organisational modes of collaboration in the generation phase. They account, indeed, on the whole sample for nearly 62%, with a growth over the time period considered, from nearly 58% in 2000 to more than 67% in 2005. This implies a clear tendency of biotech firms in cooperating with external organisations in their innovation process and particularly in the generation phase, where the quest is more relevant for innovative products (and enabling technologies) able to support the business development of top players. As a consequence, the relative weight of organisational modes of collaboration in the exploitation phase declines in the time period considered, from more than 42% in 2000 to nearly 33% in 2005.
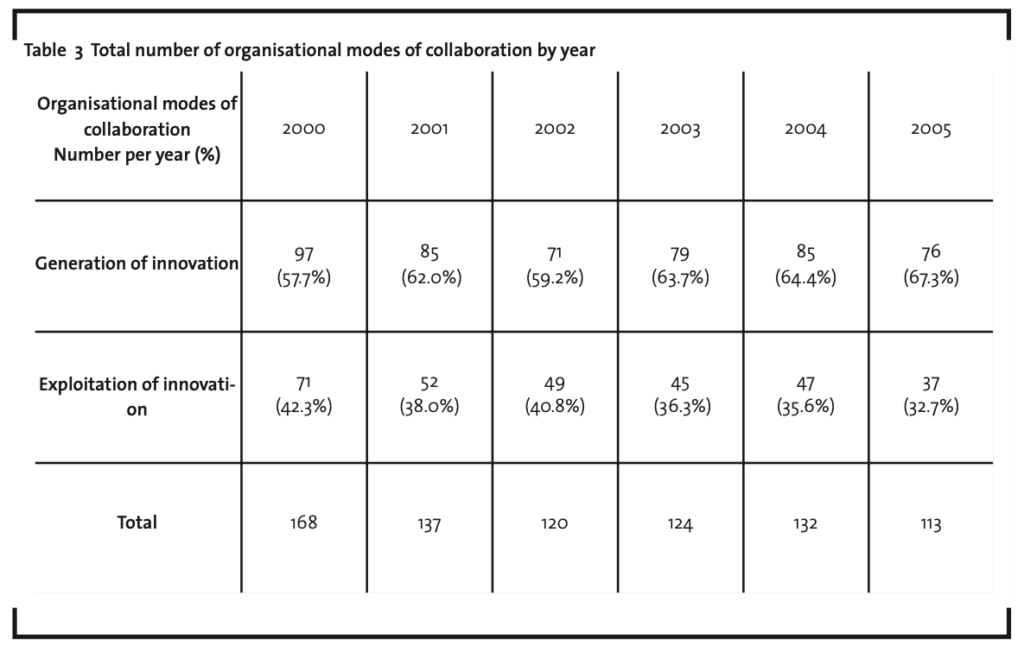
Table4 presents in more detail the different modes of collaboration in the generation and exploitation phases of innovation. First of all, it is possible to highlight the relative weight (among the modes for the generation of innovation) of in licensing, which increased from 18.6% in 2000 to more than 30% in 2005. It is interesting to notice that this growth is mostly due to a substitution of alliances with in-licensing agreements.Top players in the industry, indeed, operating as product firms (i.e. developing new drugs), have to continuously fill their product pipelines in order to remain competitive in the market and to sustain their growth against traditional pharmaceutical firms. As far as biotech firms grow and are able to use revenues from directly marketed drugs to finance their own R&D activities, they tend to adopt more in-licensing modes. Indeed, in-licensing is relatively more “expensive” than alliances but at the same time allows both to reduce the risk of competencies spill-over and to better protect intellectual property. It also ensures a better control and independence of the biotech firm in the management of the drug discovery and development process. The above remarks are further supported by the fact that the majority of in-licensing (respectively 24%, 15% and 12%) refers to products in major therapeutic areas of oncology, cardiovascular diseases, and central nervous system diseases, where competition with traditional pharmaceutical and other biotech firms is fiercest and where top players actually focus.
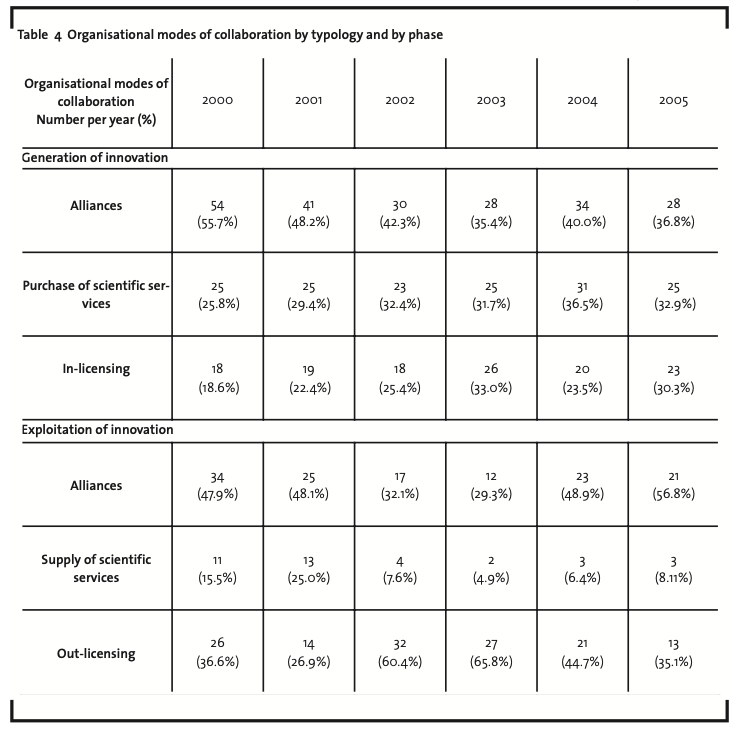
As far as organisational modes of collaboration in the exploitation phase of innovation are concerned, it is interesting to notice the relative growth of alliances (mostly co-manufacturing and co-marketing agreements). A suitable explanation for this trend is the increasing need for biotech firms (and particularly for product biotech firms) to expand their geographical coverage to reach customers on a worldwide basis. Alliances, indeed, are mostly (56% on average) signed with pharmaceutical companies, operating with a world-scale productive and distributive capacity.
An interesting up-and-down trend in the average weight can be also recognised in out-licensing (decreasing from nearly 37% in 2000 to more than 35% in 2005, but with peaks of more than 60% in 2002 and 2003). The analysis of out-licensing requires further details on therapeutic areas. In 43% of the cases, out-licensing refers to products in major therapeutic areas (oncology, cardiovascular diseases, and central nervous system diseases), whereas the remaining 57% is distributed in a plethora of minor therapeutic areas (e.g. allergy/immunology, metabolic diseases, infectious diseases, respiratory diseases, genito-urinary diseases). The determinants of the adoption of out-licensing, indeed, are rather different in the two cases. In the former cases, biotech firms adopt out-licensing as a second-best after alliances when they are not able to reach autonomously the market or are unable to find a suitable partner. In the latter cases, on the contrary, biotech firms adopt out-licensing to profit (actually in a typical Open Innovation approach) from products whose development is not coherent with their core business, i.e.with their focus in terms of therapeutic areas. A final remark on the organisational modes of collaboration for the exploitation phase concerns the declining weight of the supply of scientific services (from 15.5% in 2000 to 8.1% in 2005). This trend is again related to the natural evolution of biotech firms. In their initial stages, they are forced to supply services (particularly technological services) to create a revenue stream able to support R&D activities. Once products reach the market, revenue streams from ancillary activities becomes less relevant and biotech firms tend to concentrate their efforts in the development process of new drugs.
The empirical evidence on the organisational modes adopted by biotech firms along the phases of the drug discovery and development process supports the model developed through the panel study (and shown in Figure 2). Concerning the macro-phase of generation of innovation it is possible to highlight that:
- on average, more than 60% of the alliances for the generation of innovation are concentrated in the phase of target identification and validation. As identified in the model, indeed, in this activity the contribution of external sources of innovation is particularly relevant as they allow biotech firms to complement internal competences in basic research;
- purchase of scientific services is concentrated in the lead identification and optimisation (48%), where it is specifically concerned with the access to technological platforms for lead optimisation. The remaining part refers to clinical tests (mainly to CROs) and, only marginally (7%) to post approval activities;
- in-licensing, that represents the main tool for filling the product pipeline and increasing the rate of introduction of new drugs into the market, progressively shifted in the time period considered from pre-clinical tests (that in 2000 represented nearly 80% of cases) to clinical tests. In-licensing in phase I (and eventually in phase II) of clinical tests represented in 2005 nearly 40% of the cases. In-licensing of products that are in later phases of the process significantly reduces the risks of development. At the same time, however, in-licensing in later phases of the process is more “expensive”, as the acquirer usually pays the more the less risky the product is, and therefore only more mature firms are able to use this mode.
Concerning the macro-phase of exploitation of innovation, it is to notice that:
- nearly 50% of the alliances in this phase are related to post-approval activities,where there is a quest for expanding geographical coverage by biotech firms;
- supply of scientific services,even if quite marginal, is concentrated almost only in the preclinical and clinical (phase I) tests,where biotech firms may exploit particularly their technological base to offer support services mainly to other biotech firms;
- in the case of out-licensing, the distinction between products in major therapeutic areas versus those in minor therapeutic areas is clearly related to the phase of the process where out-licensing takes place. In particular, outlicensing for products in minor therapeutic areas concentrates mainly in pre-clinical tests (from 40% in 2000 to nearly 70% in 2005).This reduces the financial effort (and risk) for biotech firms in developing products that are out of their main business scope and,at the same time, allows firms to find an effective way to profit from these products. Out-licensing for products in major therapeutic areas, on the contrary, is even more pursued in the later phasesoftheprocess(andparticularlyinphase II and III of clinical tests,45% and 23% in 2005 up to 38% and 15% in 2000,respectively),thus highlighting the attempt of biotech firms to increasingly reach autonomously mainstream markets.
Finally, it is possible to analyse the typology of external partners involved in collaborations and their evolution along the different phases of the bio-pharmaceutical innovation process. The concept of “typology” of partners has already been discussed in the third section. However it is worth remembering here that this concept comprises both a qualitative characterisation (distinguishing between pharmaceutical firms, product biotech firms, platform biotech firms, universities and research centres) and a quantitative characterisation (distinguishing between small-medium and large companies). Analysing the organisations with which biotech firms in our sample have established collaborations, the following typologies of partners emerged:
- large pharmaceutical firms. This typology comprises traditional pharmaceutical firms, i.e. those operating in the industry since before the advent of biotechnology with a long tradition in “chemical-based” pharmacology. All pharmaceutical firms found in our database are large firms. This is not surprising, however, and for a twofold reason: on the one side, pharmaceutical firms are on average older and more mature than biotech firms and, on the other side, they represent, in the large majority of cases, the “natural” partner for large biotech firms searching to expand their geographical and/or market coverage. It seems obvious that large pharmaceutical companies fit better with the latter purpose;
- product biotech firms. Product biotech firms are those firms that have as a main business goal the development and marketing of new drugs. These firms are very similar in nature to the top players in the industry and according to their different stage of development, we found product biotech firms that are either of large or small-medium size in our databases. More in particular,80% of product biotech firms are small-medium companies,whereas the remaining 20% are large companies;
- small-medium platform biotech firms. This typology comprises biotech firms involved in the development of enabling technologies for the drug discovery and development process. It is noteworthy that only small and medium size companies of this typology are found in our database. However,this appears reasonable considering the nature of their business. The large majority of platform biotech firms, indeed, operates on a small scale, offering a set of technologies to product biotech firms in a limited geographical area (usually within an industrial cluster);
- universities and research centres. This typology comprises the other external organisations involved in the process of drug discovery and development.
Table 5 summarises the results of the analysis. In particular,for each organisational mode of collaboration the number of times (measured by the relative percentage) a given typology of partner is involved is reported.
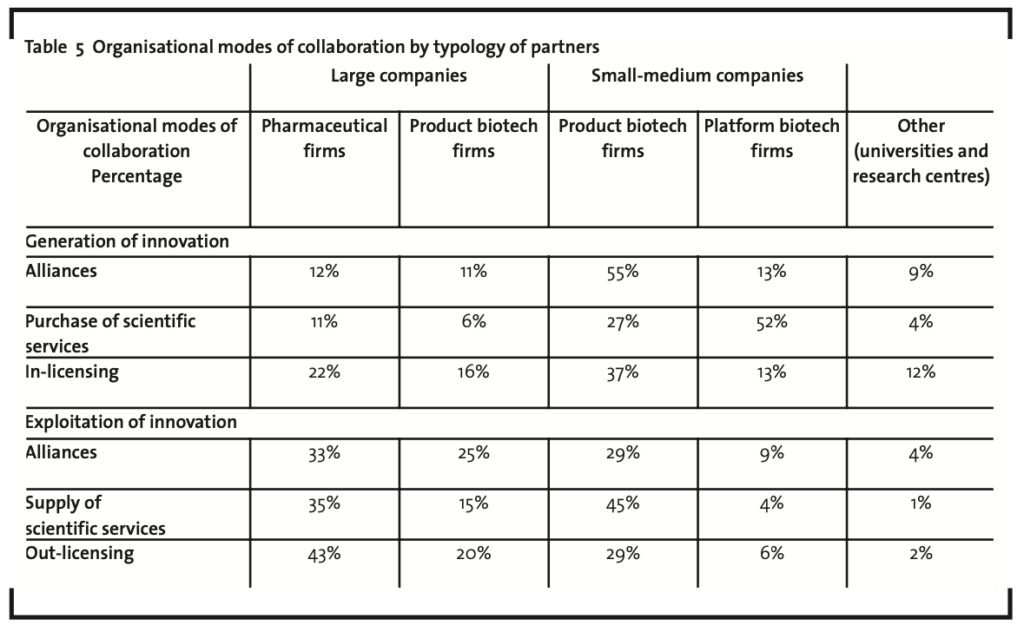
Within the macro-phase of generation of innovation, small-medium companies (and more particular small-medium product biotech companies) clearly prevail, with an average percentage of occurrence of nearly 66%. The reason behind this evidence is the willingness of top players in the industry to sustain their drug development process through accessing most innovative scientific competencies (alliances), technological assets (purchase of scientific services) and products (inlicensing). Small and medium-size companies, indeed, even if usually started around a very innovative and high potential idea, in most cases do not have the financial resources nor the complementary assets needed to sustain on their own the whole process of development. Therefore, they become an attractive partner for larger biotech companies, which can also exploit their bargaining power in setting the terms of the organisational mode of collaboration.
Further on,it is interesting to notice the relatively marginal role played by universities and research centres, which on average account for about 8% of all partners. This may appear to be in contrast to a large part of the literature (among others Chiesa,2004; Malerba and Orsenigo, 2002) claiming the pivotal role of universities and research centres in generating biotechnology innovation and in sustaining the creation of new biotech firms (academic spin-offs). In this case, however, the reason has to be found in the peculiar characteristics of the sample, including only the largest biotech firms. These companies, indeed, prefer to collaborate with other companies, which have already started the process of development of the new product (maybe with an academic origin), rather than with universities and research centres that usually conduct only very basic research. On the one side, this approach reduces the risk of the innovative process (as initial stages of development had already succeeded) and, on the other side, even if more expensive, it is viable for large companies that can exploit financial resources generated from marketed products. In the macro-phase of exploitation of innovation, large companies (particularly pharmaceutical firms) play a pivotal role, representing on average nearly 57% of total partners involved in collaborations. Looking in closer detail at the single rows of the Table 5, it is possible to highlight the following:
- in the alliances for the exploitation of innovation, as already discussed, top biotech industry players mostly need reliable partners to expand their geographical and/or market coverage, through complementing their existing market assets. Large pharmaceutical firms, which usually already operate on a worldwide basis, represent the best solution for this purpose. Otherwise, co-marketing agreements can be signed with other product biotech firms to join forces in distribution and selling activities (large product biotech firms) or to exploit a particular geographical or therapeutic focus (small-medium product biotech firms);
- in the supply of scientific services again the role of large pharmaceutical firms (and more general of large companies) appears to be of relevance. The support by these firms is usually related to clinical tests. It should be noticed, however, that small and medium product biotech firms account for the largest relative share (45%).This is mainly due to the fact that they usually offer pre-clinical tests services to third parties, thus attempting to sustain their research effort with an ancillary stream of revenues. This activity is usually abandoned once business maturity is reached (as the low percentage of large product biotech firms involved demonstrates). Finally, the marginal role of platform biotech firms, usually more focused on technology supply for initial research activities, has to be mentioned;
- in the out-licensing agreements, large pharmaceutical companies gain the “lion’s share” still exploiting their competitive advantage (that is however fast eroding) in complementary assets in respect of top biotech industry players, particularly in major therapeutic areas. Small and medium product biotech companies, on the contrary, are the best partners for out-licensing agreements that involve new drugs for those minor therapeutic areas that fall out of business scope of top industry players.
A clear pattern of evolution can be therefore recognised in the typologies of partners involved in organisational modes of collaboration. In the macro-phaseofgenerationofinnovationtheinnovative contribution of small and medium companies (both product and platform firms) is of paramount importance, whereas in the macrophase of exploitation of innovation large companies prevail exploiting their strength in existing complementary assets. This is consistent with the already discussed evolution of the organisational modes of collaboration in the two macrophases.
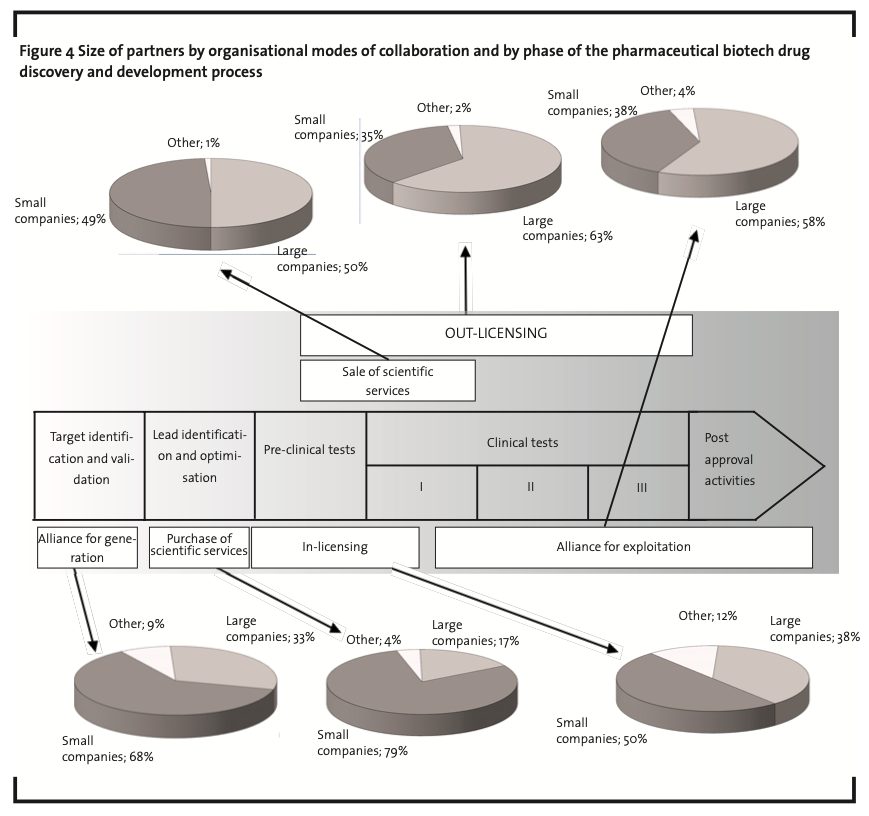
Figure 4 is a comprehensive picture of the results of the analysis and schematically represents the evolutionary pattern.It is worth mentioning that the variety of partners involved, of organisational modes adopted and their evolution along the pharmaceutical biotech drug discovery and development process are a clear example of the adoption in the industry (or at least by its top players) of the paradigm of Open Innovation.
Conclusions
The paper contributes to the on-going debate on the role of collaborations in the bio-pharmaceutical industry. In particular, it systematically and longitudinally assesses the extent and variety of organisational modes of collaboration adopted by biotech firms, the relations among different organisational modes, the phases of the drug discovery and development process, and the typologies of partners involved. Moreover, it represents one of the first attempts to study the adoption of the Open Innovation paradigm in a definite industry.
A framework of analysis has been developed through a panel study, identifying different organisational modes of collaborations and their relations with the phases of the bio-pharmaceutical innovation process.The framework has then been applied to a longitudinal empirical base including data about the collaboration of top 20 worldwide industry players, in the time period 2000-2005.
The results of the analysis allow to initially assess the framework and to discuss the determinants of the adoption of different organisational modes of collaboration and the role of different typologies of partners. In particular, the paper highlights that the peculiarities of the biotech industry (e.g.the articulation of the innovation process and its typical risk pattern, the business focus of biotech firms towards major therapeutic areas, the problems related to the management of IPRs) are crucial to analyse the pattern of evolution of organisational modes of collaboration and also represent the key to understand the typology (and particularly the size) of partners involved in collaborations. The overall picture resulting from the empirical analysis supports the idea that the biotech industry is a clear example of industrial sectors where the Open Innovation paradigm is in place.
Nevertheless some limitations of the research should be addressed in future research. In particular, it is necessary to further investigate whether and how the composition of the sample, which includes only large product biotech firms (i.e.firms developing new drugs),affects the results. It might be possible to argue, e.g. that platform biotech firms are less compelled with the need to fill their product “pipeline” and therefore have a different approach to collaborations, or that smaller firms adopt in- and out-licensing strategies that are different (or even exactly the opposite) from those of large firms.
However, the authors believe this paper represents a valuable basis for future research and managerial discussions in the field.
References
Audretsch, D.B. and Stephan P.E. (2001). Biotechnology in Europe: lessons from the USA, International Journal of Biotechnology, 3 (1/2), p.168 – 183.
Barbanti, P., Gambardella, A. and Orsenigo L. (1999). The Evolution of Collaborative Relationship Among Firms in Biotechnology, International Journal of Biotechnology , 1 (1), p. 10 – 29.
Baum, J.A.C., Calabrese, T. and Silverman B.S. (2000). Don’t go it alone: alliance network composition and startups’ performance in Canadian biotechnology, Strategic Management Journal, 21 (3), p.267 – 294. Burrill&Company, (2005). Biotech 2005. Lifesciences.
Byerlee, D., Maredia, M. and Maredia, K. (1999). Investment strategies for biotechnology in emerging research systems, paper published in the proceedings of the ICABR Conference, University of Rome “Tor Vergata”, Rome, June 17 – 19.
Chesbrough, H. (2003). Open Innovation: the new imperative for creating and profiting from technology, Harvard Business School Press, Boston.
Chesbrough, H., Vanhaverbeke, W. and West, J. (2006). Open Innovation: Researching a New Paradigm, Oxford: Oxford University Press.
Chesbrough, H. (2003). The logic of open innovation: managing intellectual property, California Management Review, 45 (3).
Chesbrough, H. (2004). Managing open innovation, Research-Technology Management, 47 (1), p. 23 – 26.
Chesbrough, H. and Crowther, A.K. (2006). Beyond hightech: early adopters of open innovation in other industries, R&D Management, 36 (3), p. 229 – 236.
Chiaroni, D., Chiesa, V., De Massis, A. and Frattini, F. (2008). The knowledge bridging role of technical and scientific services in knowledge-intensive industries, International Journal of Technology Management, (forthcoming).
Chiesa, V. (2003). La Bioindustria. Strategie competitive e organizzazione industriale nel settore delle biotecnologie farmaceutiche, ETAS, Milano.
Chiesa, V. and Toletti, G. (2004). Network of collaborations for innovation: the case of biotechnology, Technology Analysis & Strategic Management, 16 (1), p. 73 – 96. Chiesa, V. (2001). R&D strategy and organisation, Imperial College Press, London.
Chiesa, V. and Manzini, R. (1998). Organising for technological collaborations: a managerial perspective, R&D Management, 28 (3), p. 199 – 212.
Chiesa, V. and Chiaroni, D. (2004). Industrial clusters in biotechnology. Driving forces,evelopment processes and management practices, Imperial College Press, London.
DeCarolis, M. and Deeds, D. (1999). The impact of stocks and flows of organizational knowledge on firm performance: an empirical investigation of the biotechnology industry, Strategic Management Journal, 20 (10), p. 953 – 968.
Deeds, D.L. and Hill, C.W.L. (1996) Strategic alliances and the rate of new product development: an empirical study of entrepreneurial biotechnology firms, Journal of Business Venturing, 11 (1) p. 41 – 55.
Dussauge, P. and Garrette, B. (2000). Alliances versus acquisitions: choosing the right option, European Management Journal, 18 (1), p. 63 – 69. Ernst&Young, (2004). Beyond Borders 2004, Ernst&Young, London.
Ernst&Young, (2005). Beyond Borders 2005, Ernst&Young, London.
Ernst&Young, (2006). Beyond Borders 2006, Ernst&Young, London.
European Commission Recommendation, (2002). Modifications to Recommendation 96/280/CE with reference to definition of small and medium firms, Bruxelles, June, 25.
Fetterhoff, T.J. and Voelkel, D. (2006). Managing open innovation in biotechnology, Research-Technology Management, 49 (3), p. 14 – 18.
Fontes, M. (2003). Distant networking? The ‘out-cluster’ strategies of new biotechnology firms, in Green, K., Miozzo, M. and Dewick, P., (eds.) Technology, Knowledge and the Firm: Implications for Strategy and Industrial Change, Edward Elgar, London.
Gassmann, O. and Reepmeyer, G. (2005). Organizing pharmaceutical innovation: from science-based knowledge creators to drug-oriented knowledge brokers, Creativity and Innovation Management, 14 (3), p. 233 – 245.
Gulati, R. (1998) Alliances and networks, Strategic Management Journal, 19 (4), p. 293 – 317. Helfat, C.E. (1997). Know-how and asset complementarity and dynamic capability accumulation: the case of R&D, Strategic Management Journal, 18 (5), p. 339-360.
Huston, L. and Sakkab, N. (2006). Connect and Develop. Inside Procter & Gamble’s new model for innovation, Harvard Business Review, 85 , p. 58 – 66.
Kirschbaum, R. (2005). Open innovation in practice, Research-Technology Management, 48 (4), p. 24 – 28.
Lane, P.J. and Lubatkin, M. (1998). Relative absorptive capacity and interorganizational learning, Strategic Management Journal, 19 (5), p. 461 – 478.
Laroia, G. and Krishnan, S. (2005). Managing drug discovery alliances for success, Research-Technology Management, 48 (5), p. 42 – 50.
Lerner, J., Shane, J. and Tsai, A. (2003). Do equity financing cycles matter? evidence from biotechnology alliances, Journal of Financial Economics, 67 (3), p. 441 – 446. Liebeskind, J.P., Oliver, A.L., Zucker, L. and Brewer, M. (1996).
Social networks, learning, and flexibility: sourcing scientific knowledge in new biotechnology firms, Organization Science, 7 (4), p. 375 – 387.
Madhok, A. and Osegowitsch, T. (2000). The International Biotechnology Industry: A Dynamic Capabilities Perspective, Journal of International Business Studies, 31 (2), p. 325 – 335.
Malerba, F. and Orsenigo, L. (2002). Innovation and market structure in the dynamics of the pharmaceutical industy and biotechnology: towards a history friendly model, Industrial and Corporate Change, 11 (4), p. 667 – 703.
McKelvey, M., Alm, H. and Riccaboni, M. (2003). Does colocation matter for formal knowledge collaboration in the Swedish biotechnology-pharmaceutical sector?, Research Policy, 32 , p. 483 – 501.
Muffatto, M. and Giardina, G. (2003). Innovazioni nei Processi di Ricerca in Campo Farmaceutico, Economia & Management, 6 , p. 107 – 121.
Niosi, J. (2003). Alliances are not enough explaining rapid growth in biotechnology firms, Research Policy, 32 (5), p. 737 – 750.
Owen-Smith, J., Riccaboni, M., Pammolli, F. and Powell, W.W. (2002). A comparison of U.S. and European University-industry relations in the life sciences, Management Science, 48 (1), p. 24 – 43.
Powell, W.W. (1998). Learning from collaboration: knowledge and networks in the biotechnology and pharmaceutical industries, California Management Review, 40 (3), p. 228 – 240.
Powell, W.W., Koput, K.W. and Smith-Doerr, L. (1996). Interorganizational collaboration and the locus of innovation: networks of learning in biotechnology, Administrative Science Quarterly, 41 (1), p. 116 – 145.
Powell, W.W., Koput, K.W., Bowie, J.I. and SmithDoerr, L. (2002). The spatial clustering of science and capital: accounting for biotech firm-venture capital relationships, Regional Studies, 36 (3), p. 291 – 305.
Salman, N. and Saives, A.L. (2005). Indirect networks: an intangible resource for biotechnology innovation, R&D Management, 35 (2), p. 203 – 215.
van de Vrande, V., Lemmens, C. and Vanhaverbeke, W. (2006). Choosing governance modes for external technology sourcing, R&D Management, 36 (3), p. 347 – 363.
West, J., Vanhaverbeke, W. and Chesbrough, H. (2006). Open Innovation: a Research Agenda, in Chesbrough, H., Vanhaverbeke, W. and West, J. (eds.), Open Innovation: Researching a New Paradigm, Oxford: Oxford University Press, p. 285 – 307.