The role of hydrogen in the process industries – implications on energy infrastructure
Abstract
The industry sector is a significant consumer of energy and emitter of greenhouse gas emissions. Within this sector, the highest energy demand and emissions are caused by the process industries due to the need to chemically transform feedstocks and raw materials into basic chemicals and materials. New processes that include the use of renewable energies along the value chain are necessary. For the case of ammonia production, several technical process options to produce the required hydrogen feedstock are in principle available to adapt existing European ammonia plants. Each possible technical plant configuration has a significant impact on the respective infrastructure required. The size of the impact is calculated for hydrogen production using the following process options: water electrolysis onsite (with continuous and fluctuating electricity supply) and offsite, conventional production with CCS, and methane pyrolysis. Each of these technical options can only be applied if the respective infrastructure ensures a secure supply. This interdependency between infrastructure built-up and process options limits the speed and type of implementation of new technical options.
1 Introduction
Anthropogenic greenhouse gas emissions from fossil fuels are the main culprit for climate change observed after the onset of industrialization. Significant contributions come from the energy and industry sectors as well as transport and households. The industry sector was responsible in 2021 for 166.7 EJ, which corresponded to around 38 % of the overall energy consumption of 439.1 EJ (IEA, 2022), and emitted 9,136 Mt CO2, which amounted to around 25 % of the global CO2 emissions of 36,639 Mt CO2. Most of the energy consumption and emissions are caused by the so-called energy- and emission-intensive process industries, i. e. iron and steel, chemicals, non-ferrous metals, pulp and paper, cement, glass, and ceramics.
The common foundation of these industries is the chemical and physical transformation of raw materials and feedstocks into basic materials for downstream processing and manufacture. The actual transformation itself is the most energy-intensive step. Therefore, industrial transformation towards a goal of greenhouse neutrality can only be successful if the central processes of these industry sectors are addressed by new and more sustainable alternatives. Additionally, these industries are governed by economy of scale and their existing processes are highly energy efficient. The required chemical and physical transformations have a significant thermodynamic energy demand therefore these industries will remain large energy consumers, albeit their processes need to change to allow renewable energy carriers rather than fossil ones. Furthermore, besides technical challenges, new processes based on renewable energies tend to be significantly more costly than existing processes based on fossil fuels.
In most cases, direct electrification might not be suitable and indirect electrification will play a significant role. Not necessarily will the new processes follow the same economy-of-scale or efficiency gains. In some cases, these new processes might actually consume more energy than existing ones. More importantly, however, the industrial transformation will proceed over some time in which old and new processes compete.
Hydrogen is a universal energy carrier that can fulfill different functions in the energy system. It can also be used on the merits of its chemical properties, especially within energy intensive industries, most notably in iron & steel production and the chemical industry. However, implementing hydrogen technology in the process industries has a significant impact on the required energy and feedstock infrastructure to the extent that existing infrastructures becoming obsolete, need to be significantly expanded or even new infrastructure needs to be built.
Ammonia production is second of the largest chemical processes in existence, only surpassed by the production of high-value chemicals, especialle ethylene, and propylene. It is also the largest hydrogen consumer globally. Current global production reaches 184 Mt/a (IFA, 2023) in 2021. Its product is indispensable for chemical fertilizer production as well as an entrance vector for other chemical value chains. It is also a significant contributor to greenhouse gas emissions, estimated to be responsible for 1.3 % of overall global emissions (IEA, 2021). These emissions originate from steam reforming of natural gas, autothermal reforming, partial oxidation, or gasification of feedstock to generate hydrogen for subsequent Haber-Bosch synthesis. A previous study investigated the impact of ammonia production options within the trilateral region (Ausfelder et al., 2020) between Belgium, the Netherlands, and Germany. It identified possible infrastructure requirements as a major aspect shaping industrial transformation.
2 Main Part
A study funded by Fertilizers Europe was carried out by DECHEMA (Ausfelder et al., 2022) to establish the greenhouse gas mitigation potential for the European ammonia production up to 2030 by substituting hydrogen production in existing plants in Europe with suitable alternative processes. The following analysis is based on this study.
Ammonia production in Europe is almost entirely based on natural gas as feedstock. Around two thirds of ammonia production is used to produce nitrates, one third goes into urea production. The latter requires some of the CO2 from the steam reforming step as input to react with ammonia.
There are several process options to convert existing ammonia plants towards less greenhouse gas intensive ammonia production. To which extent a given option is feasible for a given site depends strongly on the overall process configuration at the site, e. g. if the downstream processing is urea or nitric acid production.
Since the emission-intensive step is the production of hydrogen from natural gas or other fossil feedstock, hydrogen needs to be provided in a less greenhouse gas intensive way. There are several possible pathways for substituting the current ammonia production with processes that generate less greenhouse gas emissions.
Hydrogen can be co-produced with oxygen via water electrolysis. Depending on the electricity used, it can be labelled green when only renewable electricity is used. If photovoltaics or wind turbines are used, the result is in general a fluctuating and intermitting production of hydrogen. It is labelled yellow when grid electricity is used, which allows for continuous production but with specific emissions according to the electric grid energy mix.
Electrolytic hydrogen production can be done onsite or off site. Whichever solution is chosen, it has a significant impact on the infrastructure requirement: enhanced electrical connectivity for the former, new hydrogen transport infrastructure for the latter.
Substituting up to 10% of grey hydrogen of existing ammonia production by green hydrogen is assumed to be compatible with current existing production plants.
Low-emission hydrogen can also be generated in conjunction with carbon dioxide capture and storage (CCS) as so-called blue hydrogen. Ammonia is a special case for blue hydrogen since the conventional ammonia production requires separation of CO2 from hydrogen anyway, i. e. the capture process is already part of the overall process design. Currently, process CO2 is used for urea production, sold for external uses, e. g. in the food industry or just released to the atmosphere.
With respect to blue hydrogen and ammonia, rather than having the CO2-capture and storage process and transporting hydrogen to the ammonia plant, it is more compatible with the existing industrial infrastructure to produce hydrogen onsite and transport the captured CO2 to its storage place. Therefore, converting a hydrogen transport problem to a CO2-transport challenge, insofar as a CO2-infrastructure to a CCS site would enable ammonia production with limited interference to make use of blue hydrogen.
Finally, methane pyrolysis offers another pathway for hydrogen generation from natural gas without releasing greenhouse gas emissions in the process. Turquoise hydrogen is produced together with solid carbon and without CO2 being released to the atmosphere.
Notwithstanding any site-specific constraints in relation to up- or downstream processes, it is important to realize that any option requires its own infrastructure configuration to be applied at the site to guarantee security of supply and thereby a vital precondition for production. These infrastructure requirements differ both qualitatively as well as quantitatively from the demands of the existing European ammonia plants.
For comparison, infrastructure demands are calculated for an “average European ammonia plant” with a production capacity of 500,000 tNH3/y. An overview of different process configurations and the required infrastructure is shown in figure 1 for gases and in figure 2 for electrical transmission.
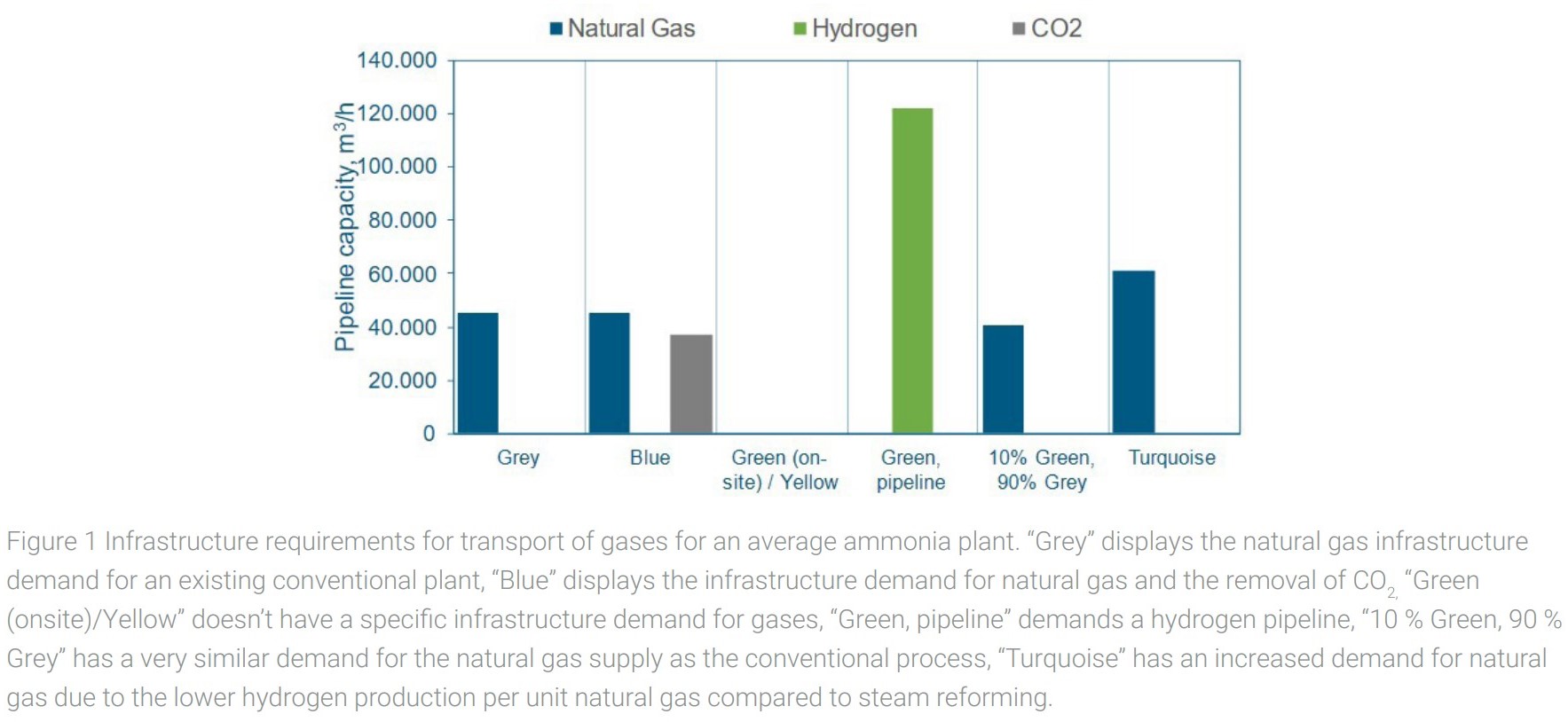
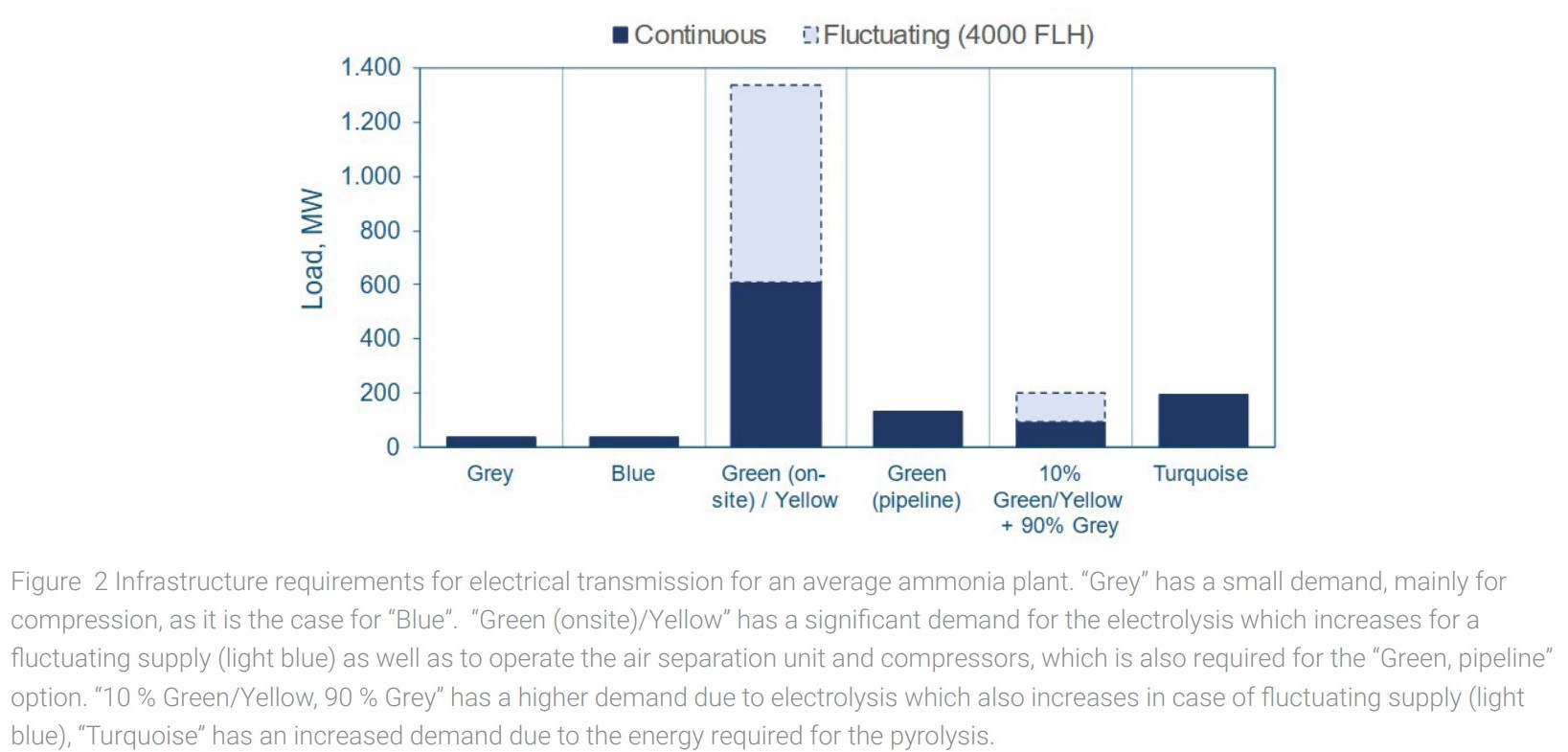
Existing grey ammonia plants have a significant demand for natural gas and are usually connected to the natural gas transport grid. Electricity demand is secondary since the main process is based on natural gas and electricity is required for compression, pumps, process control etc.
In case of a transformation towards using blue hydrogen, produced onsite as detailed above, an additional CO2-pipeline is required with the volume transport capacity of around 80 % of the existing natural gas connection. There is no significant change in the required electric load.
Completely replacing current hydrogen production with electrolysis onsite via continuous renewable (green) or grid (yellow) electricity supply would make the natural gas supply obsolete but require an 18-fold increase of the existing electrical connection.
If fluctuating (4,000 full load hours) renewable electricity instead of continuous electricity is used, the capacity factor for the electrical connection rises to 39.
Offsite production of hydrogen can be supplied by a dedicated hydrogen pipeline with a volumetric capacity of around 2.7 times the existing natural gas pipeline, while 4 times the electricity of the conventional process will be required, specifically to power an air separation unit to provide nitrogen.
Turquoise hydrogen requires both, an expansion of natural gas pipeline capacity by around a factor of 1.3 and expansion of electrical capacity by a factor of 6 compared to the existing plants.
3 Summary and Conclusion
Ammonia production is globally one of the most energy- and emission-intensive industrial processes. There are several less emission-intensive technical process alternatives available for both, greenfield and brownfield implementation. In comparison to existing ammonia plants, these technical options require either significant expansion of existing infrastructures, construction of new infrastructures and may render obsolete some existing infrastructures.
Possible industrial transformation of the European ammonia industry therefore not only depends on technical options for more sustainable processes but crucially on the availability of infrastructures. Both the choice of options to implement and the speed implementation is interdependent and limited by infrastructure development, which in turn determines the mitigation potential of the industrial transformation.
While the chosen example of ammonia production might be especially striking through the size of an average ammonia plant, the basic challenge is similar for all energy- and emission-intensive industrial processes within the process industries. Industrial transformation and infrastructural development are critically interdependent and need to be considered in conjunction.
From the perspective of a given industrial site, investment decisions on new processes will depend crucially on the expected local infrastructure development. On the other hand, transformation of the main industrial processes leads to such a significant change in energy and feedstock infrastructure demand, that it needs to be addressed proactively by the site operator responsible towards the respective infrastructure providers to allow a smooth and efficient transition and to avoid delays or involuntary production shutdowns.
There is also a risk of decohesion within the European Union or even within member states as a function of infrastructure development since new infrastructures might not be available at all production sites at the same time.
References
Ausfelder, F., van de Beek, F., Bhardwaj, R., Graf, F., Meinke-Hubeny, F., Lodewijks, P., Müller, S. A., Rijkers, M., Perez Sanchez, D., Ruf, J., (2020): Infrastructure Challenges Caused by Industrial Transformation to Achieve Greenhouse Gas Neutrality. Ammonia Production in the Antwerp-Rotterdam-Rhine-Ruhr Area, Chemie Ingenieur Technik, 93 (3), pp. 373-379.
Ausfelder, F., Herrmann, E. O., López González, L. F., (2022): Perspective Europe 2030 – Technology options for CO2-emission reduction of hydrogen feedstock in ammonia production, DECHEMA, Frankfurt.
International Energy Agency (IEA), 2021. Ammonia Technology Roadmap CC BY-NC 3.0 IGO.
International Energy Agency (2022), World Energy Outlook 2022, IEA. License: Creative Commons Attribution CC BY-NC-SA 4.0.
International Fertilizer Association (2023), IFASTAT, 2.1 Regional Overview by Product Ammonia Production in 2011, available at https://www.ifastat.org/databases/graph/2_1, accessed 11 April 2023.